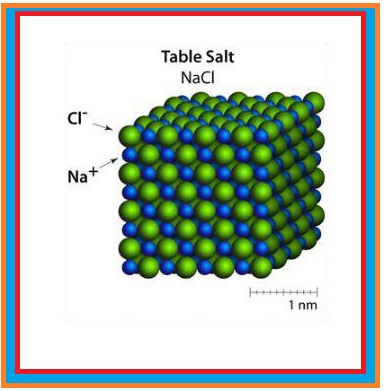
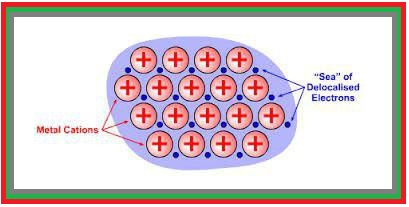
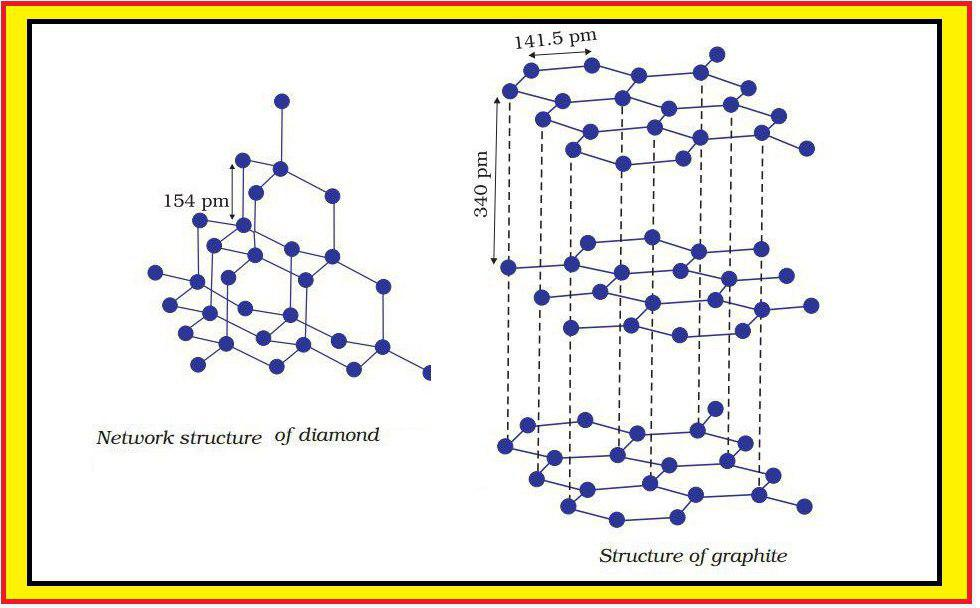
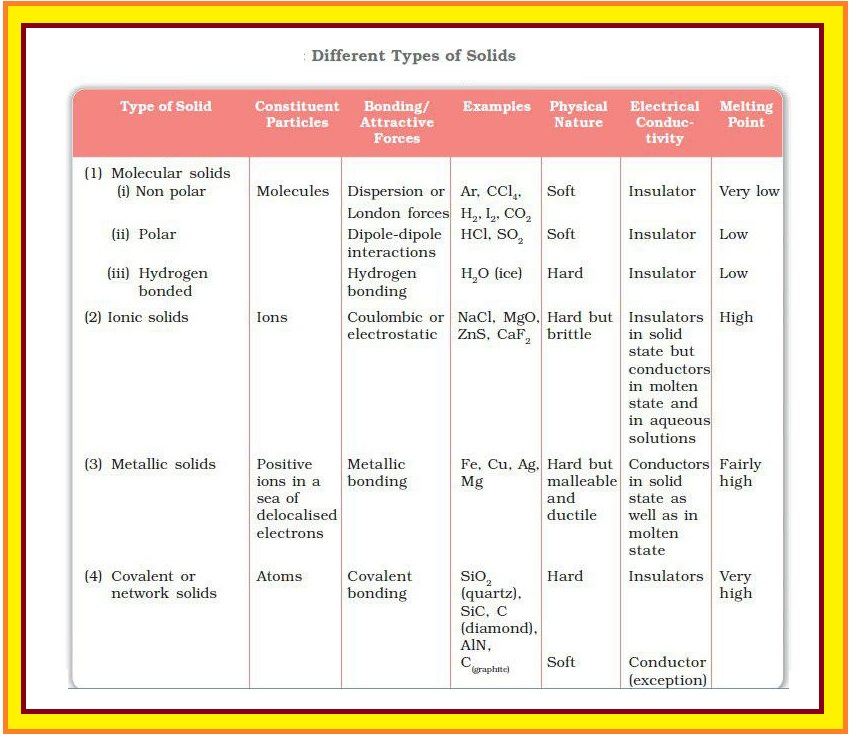
Molecular Solids :
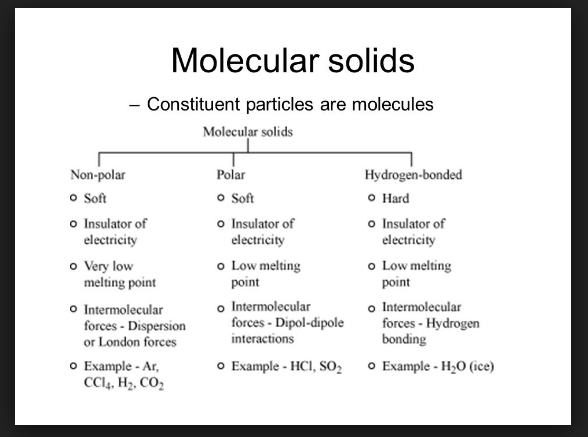
`color{green}("Molecules are the constituent")` `color{green}(" particles of molecular solids. Types of molecular solids :")`
(a) `color{green}("Non- polar molecular solids :")`
(i) These solids comprise of either atoms or the non-polar molecules.
(ii) e.g. `color{red}(Ar, He, H_2, Cl_2, I_2)` etc.
(iii) The atoms or molecules are held by weak dispersion forces or London forces.
(iv) Have low melting points.
(v) Exists in liquid or gaseous state at room temperature and pressure.
(b) `color{green}("Polar Molecular Solids :")`
(i) Polar molecules are the constituent of solids.
(ii) e.g. `color{red}(HCl, SO_2, NH_3)` etc.
(iii) Molecules are held together by dipole-dipole interactions.
(iv) Soft and non-conductors of electricity.
(v) Melting point is higher than non-polar molecular solids.
(vi) Gaseous and liquids under room temperature and pressure.
(c) `color{green}("Hydrogen Bonded Molecular Solids :")`
(i) In such solids there is covalent bond between `color{red}(H)` and `color{red}(F, O)` or `color{red}(N)` atoms e.g. `color{red}(H_2O)`
(ii) They are non-conductors of electricity.
(iii) Volatile liquids or soft solids at room temperature and pressure.
(a) `color{green}("Non- polar molecular solids :")`
(i) These solids comprise of either atoms or the non-polar molecules.
(ii) e.g. `color{red}(Ar, He, H_2, Cl_2, I_2)` etc.
(iii) The atoms or molecules are held by weak dispersion forces or London forces.
(iv) Have low melting points.
(v) Exists in liquid or gaseous state at room temperature and pressure.
(b) `color{green}("Polar Molecular Solids :")`
(i) Polar molecules are the constituent of solids.
(ii) e.g. `color{red}(HCl, SO_2, NH_3)` etc.
(iii) Molecules are held together by dipole-dipole interactions.
(iv) Soft and non-conductors of electricity.
(v) Melting point is higher than non-polar molecular solids.
(vi) Gaseous and liquids under room temperature and pressure.
(c) `color{green}("Hydrogen Bonded Molecular Solids :")`
(i) In such solids there is covalent bond between `color{red}(H)` and `color{red}(F, O)` or `color{red}(N)` atoms e.g. `color{red}(H_2O)`
(ii) They are non-conductors of electricity.
(iii) Volatile liquids or soft solids at room temperature and pressure.