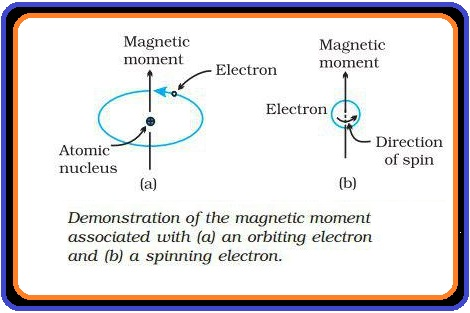
(i) `text(Paramagnetism)` : (a) Weekly attracted by a magnetic field.
(b) Magnetised in the magnetic field in same direction.
(c) Lose magnetism in the absence of magnetic field.
(d) It is due to the presence of one or more unpaired electrons.
(e) e.g. `O_2`, `Cu^(+2)`, `Fe^(+3)`, `Cr^(+3)` etc.
(ii) `text(Diamagnetism)` : (a) Weekly repelled by a magnetic field.
(b) Weekly magnetised in a magnetic field in opposite direction.
(c) Shown by substances in which all electrons are paired because pairing of electrons cancels their magnetic moments.
(d) e.g. `H_2O`, `NaCl` and `C_6H_6`
(iii) `text(Ferromagnetism)` : (a) Strongly attracted by magnetic field.
(b) Can be magnetised permanently.
(c) In solid state, the metal ions of ferromagnetic substance are grouped together into small regions called domains and each domain acts as a tiny magnet.
(d) In the absence of magnetic field, domains are randomly oriented and their magnetic moments get cancelled.
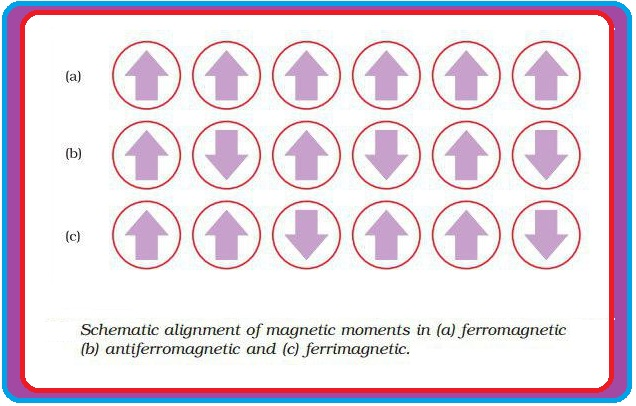
(e) In the presence of magnetic field, domains are oriented in the direction of magnetic field and a strong magnetic effect is produced (Fig 1.32 a).
(f) e.g. `Fe`, `Co`, `Ni`, `CrO_2`, gadolinium.
(iv) `text(Anti ferromagnetism)` : (a) In this, domains are oppositely oriented and cancel out each others magnetic moment (Fig 1.32 b).
(b) e.g `MnO`
(v) `text(Ferrimagnetism)` : (a) In this, magnetic moments of the domains in the substance are aligned in parallel and anti-parallel directions in unequal numbers (fig 1.32 c).
(b) Weekly attracted by magnetic field as compared to ferromagnetic substances.
(c) Loses ferrimagnetism on heating and become paramagnetic.
(d) e.g. `Fe_3O_4` (magnetite) and ferrites like `MgFe_2O_4` and `ZnFe_2O_4`.
(i) `text(Paramagnetism)` : (a) Weekly attracted by a magnetic field.
(b) Magnetised in the magnetic field in same direction.
(c) Lose magnetism in the absence of magnetic field.
(d) It is due to the presence of one or more unpaired electrons.
(e) e.g. `O_2`, `Cu^(+2)`, `Fe^(+3)`, `Cr^(+3)` etc.
(ii) `text(Diamagnetism)` : (a) Weekly repelled by a magnetic field.
(b) Weekly magnetised in a magnetic field in opposite direction.
(c) Shown by substances in which all electrons are paired because pairing of electrons cancels their magnetic moments.
(d) e.g. `H_2O`, `NaCl` and `C_6H_6`
(iii) `text(Ferromagnetism)` : (a) Strongly attracted by magnetic field.
(b) Can be magnetised permanently.
(c) In solid state, the metal ions of ferromagnetic substance are grouped together into small regions called domains and each domain acts as a tiny magnet.
(d) In the absence of magnetic field, domains are randomly oriented and their magnetic moments get cancelled.
(e) In the presence of magnetic field, domains are oriented in the direction of magnetic field and a strong magnetic effect is produced (Fig 1.32 a).
(f) e.g. `Fe`, `Co`, `Ni`, `CrO_2`, gadolinium.
(iv) `text(Anti ferromagnetism)` : (a) In this, domains are oppositely oriented and cancel out each others magnetic moment (Fig 1.32 b).
(b) e.g `MnO`
(v) `text(Ferrimagnetism)` : (a) In this, magnetic moments of the domains in the substance are aligned in parallel and anti-parallel directions in unequal numbers (fig 1.32 c).
(b) Weekly attracted by magnetic field as compared to ferromagnetic substances.
(c) Loses ferrimagnetism on heating and become paramagnetic.
(d) e.g. `Fe_3O_4` (magnetite) and ferrites like `MgFe_2O_4` and `ZnFe_2O_4`.