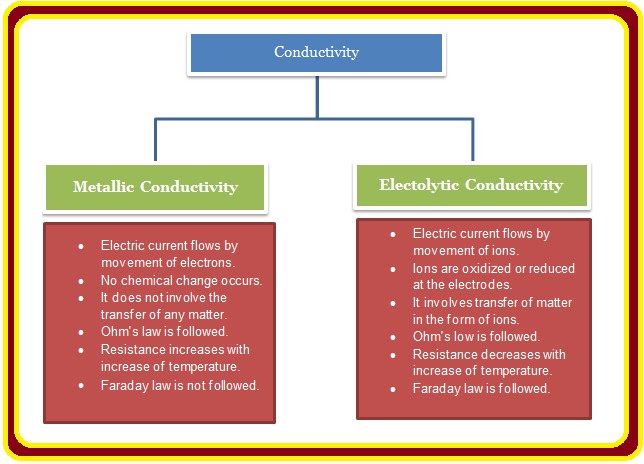
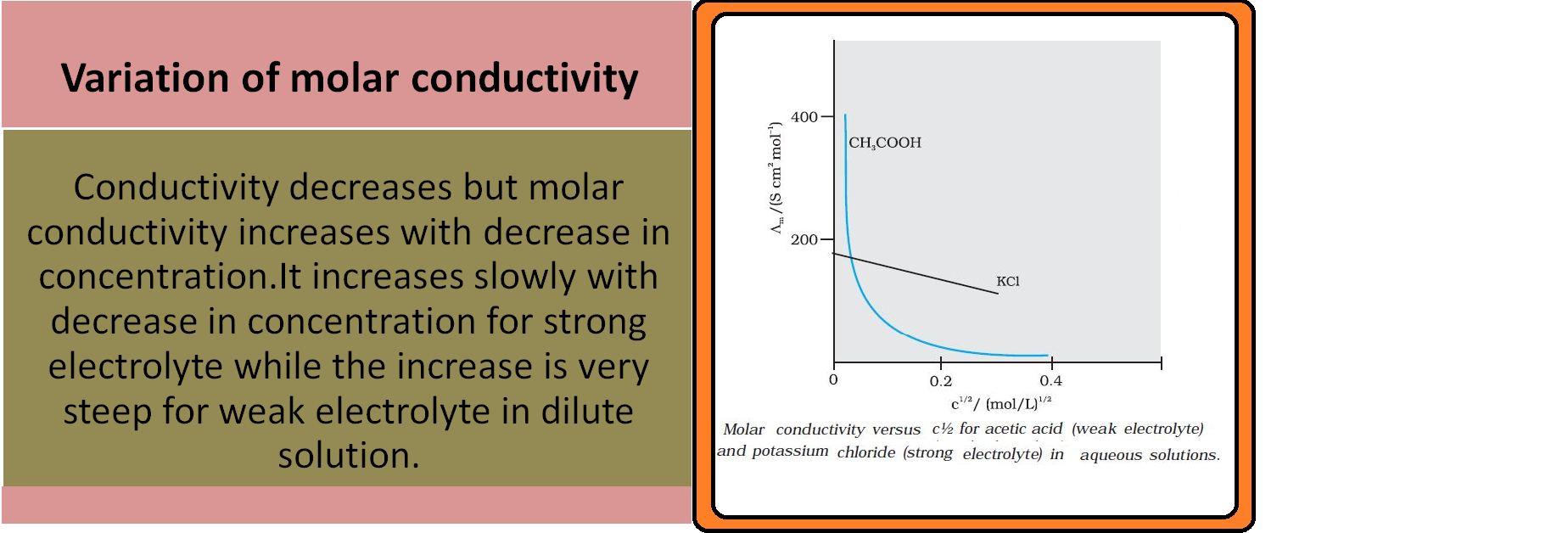
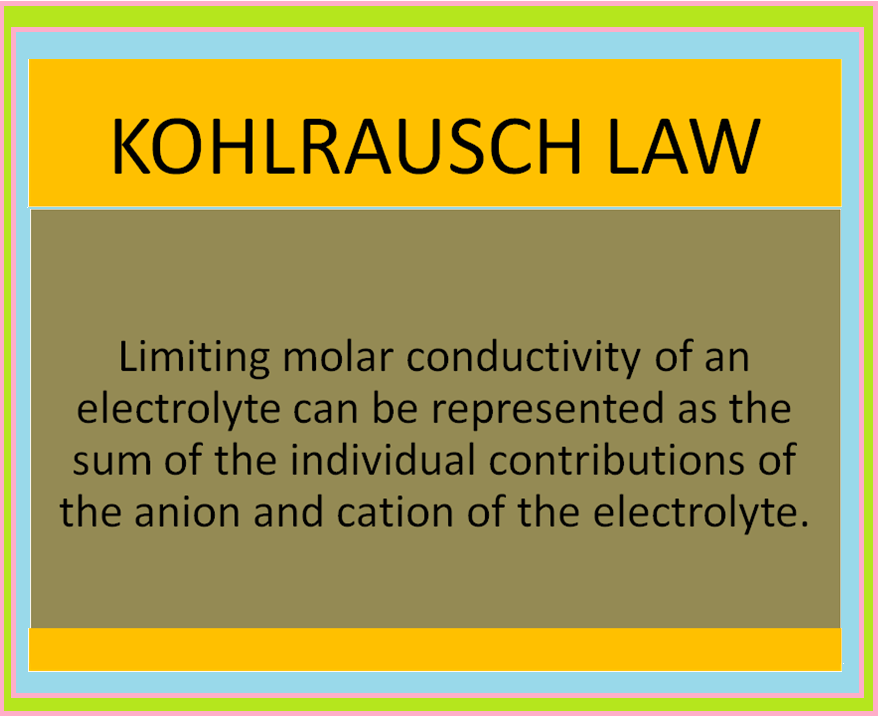
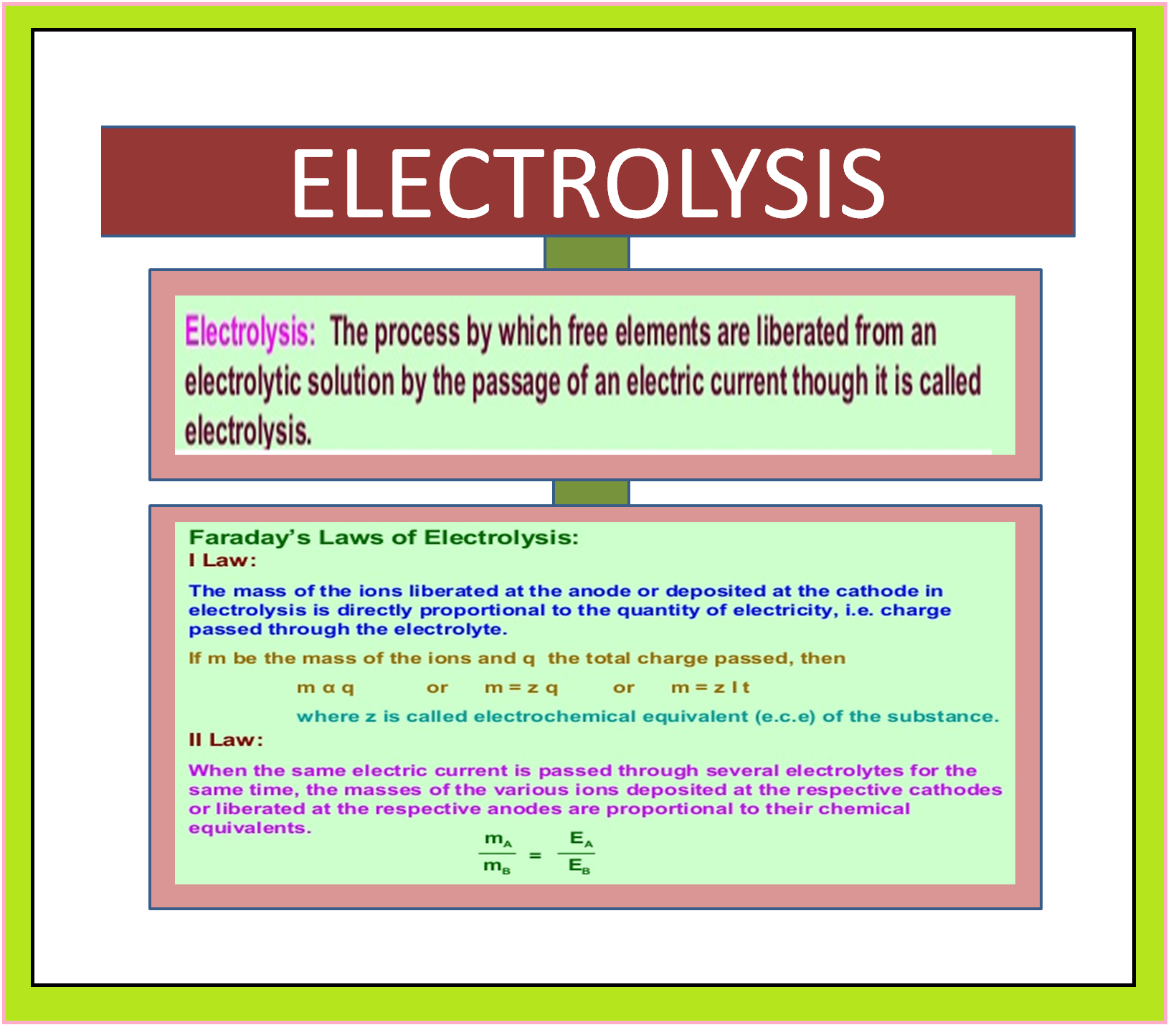
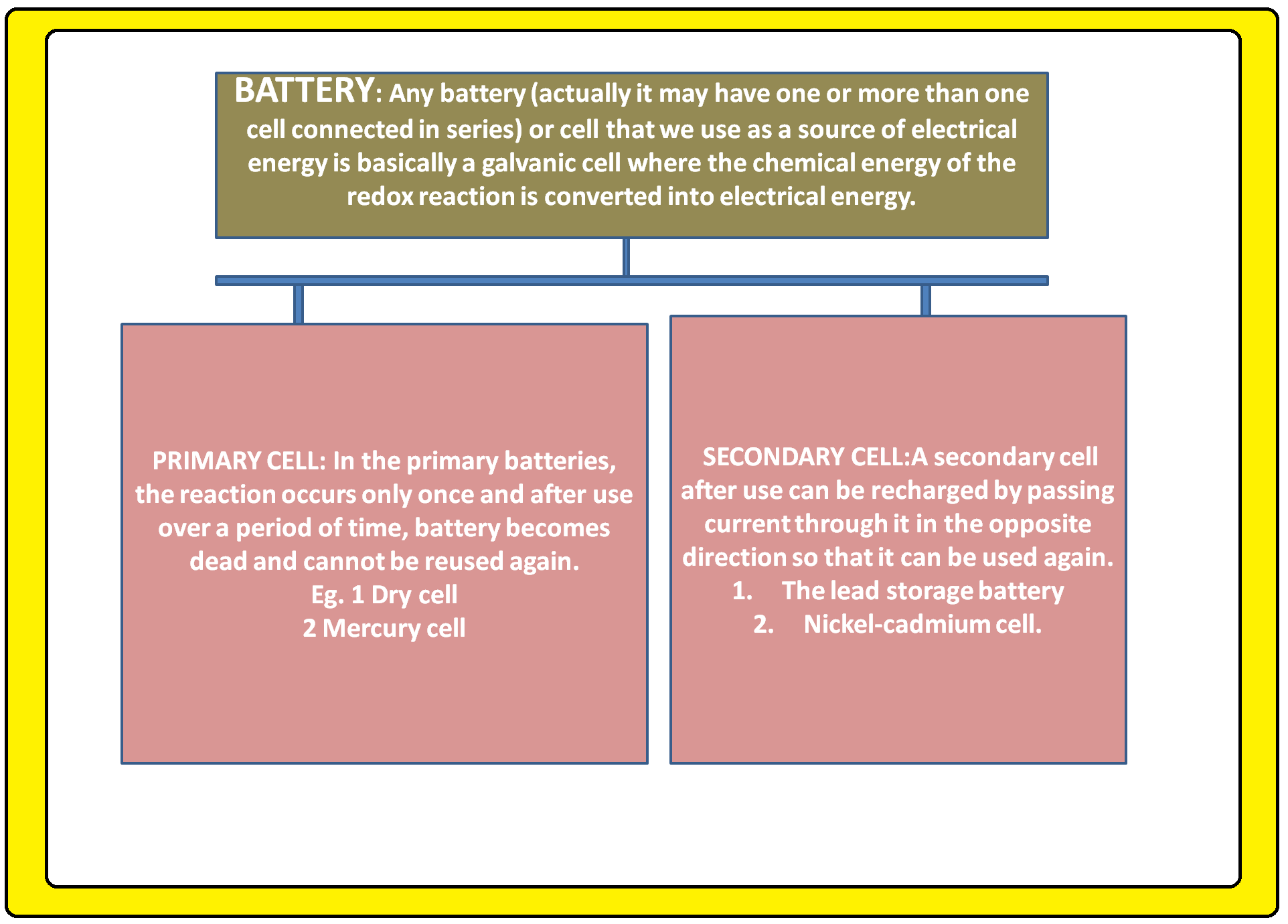
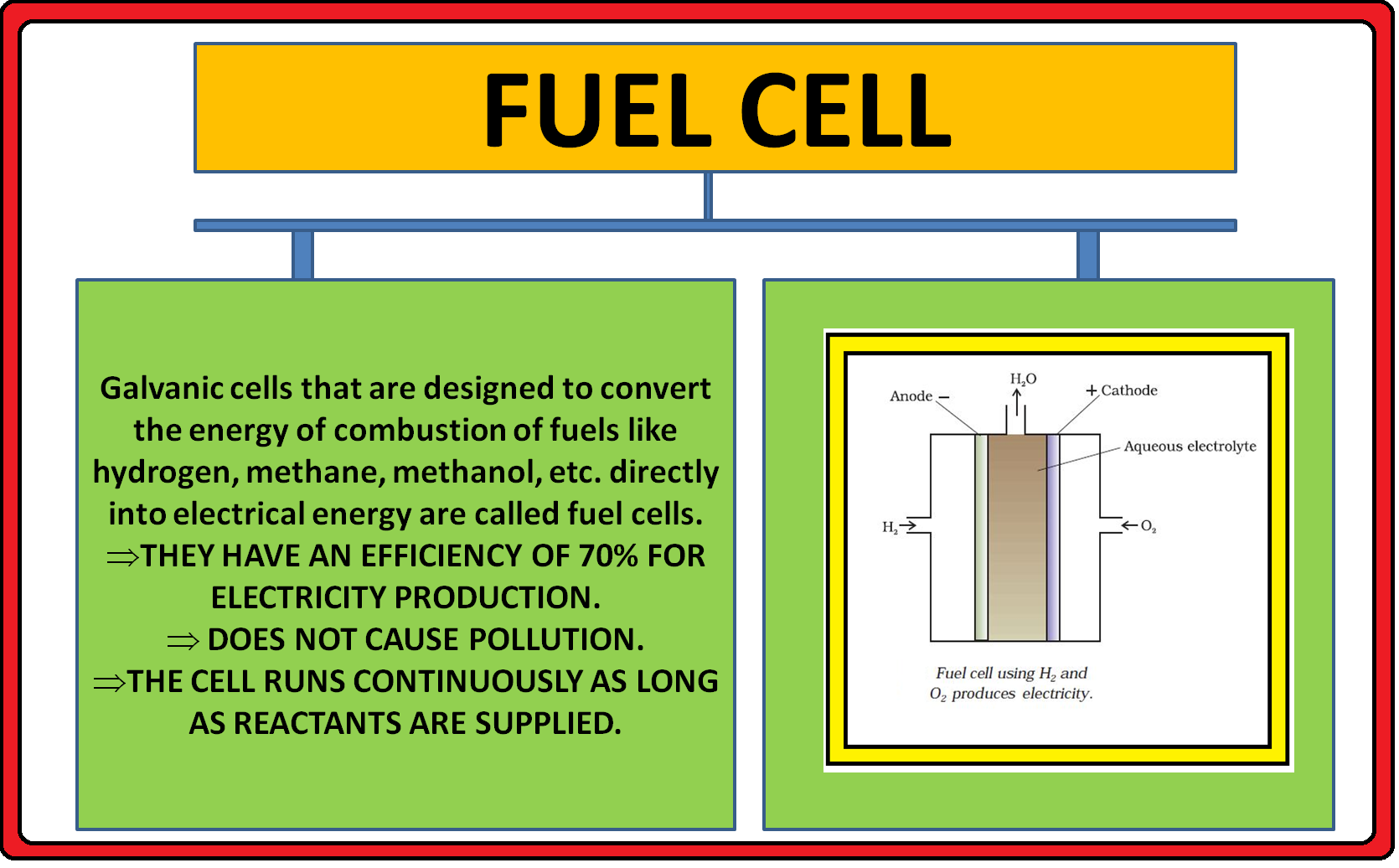
ELECTROCHEMICAL CELL
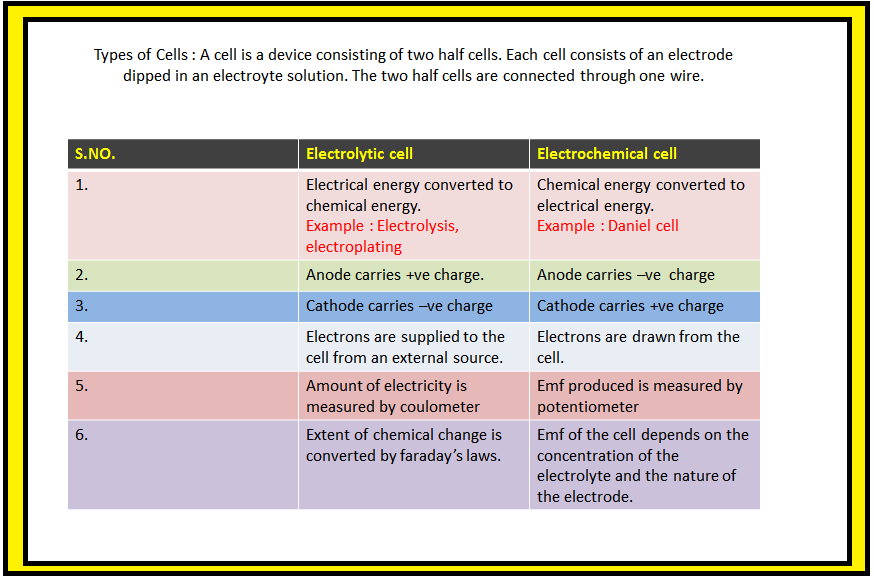
`=>` `color{green}("Electrochemistry") :` It is the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations.
`=>` `color{green}("Galvanic cell") :` A galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical energy.
`=>` `color{green}("Galvanic cell") :` A galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical energy.