INTRODUCTION
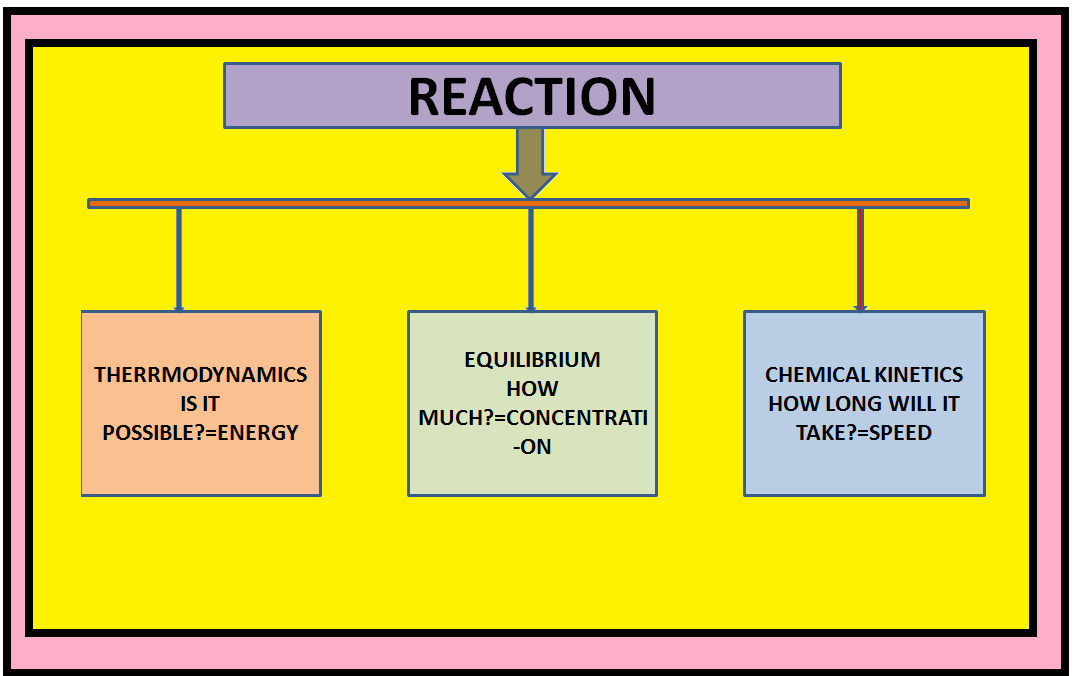
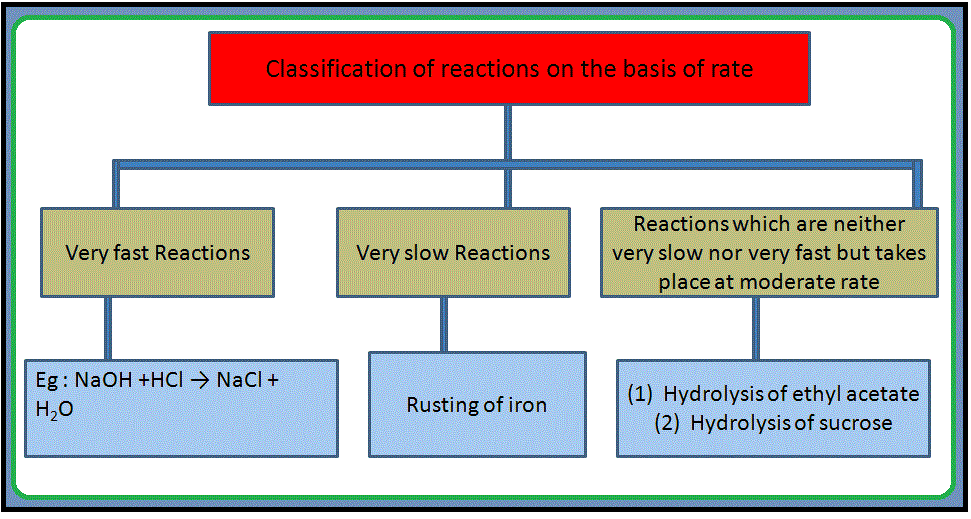
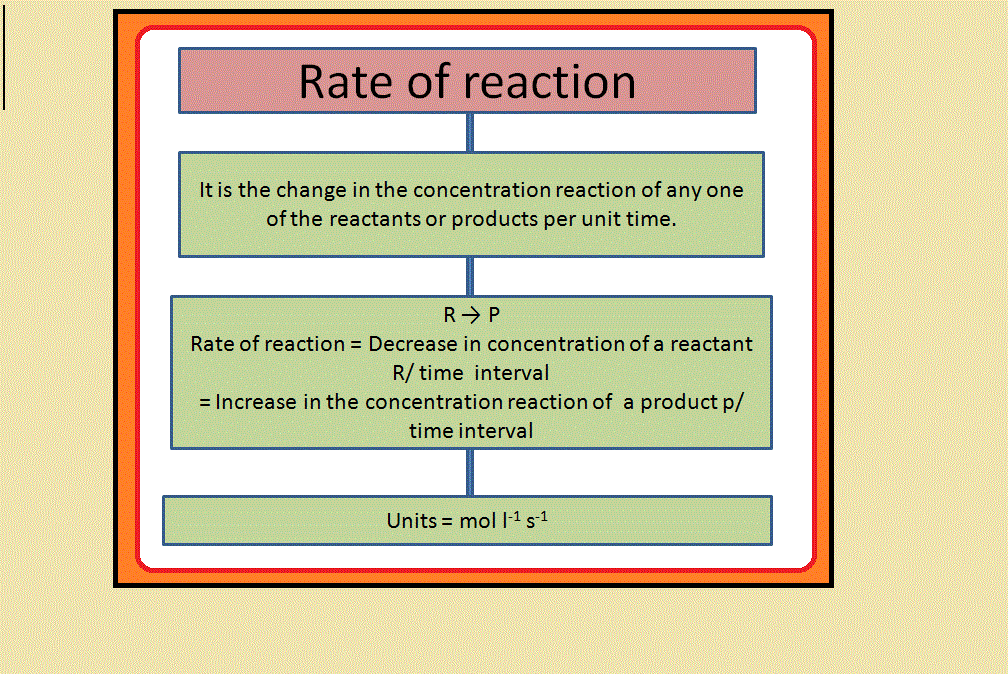
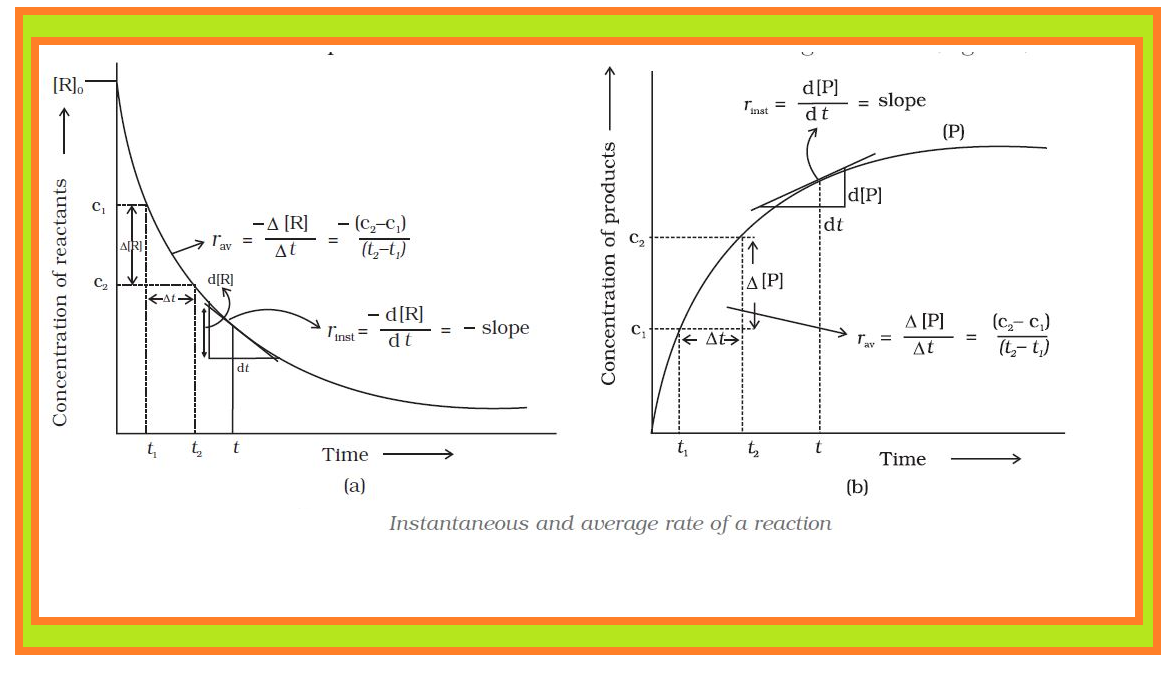
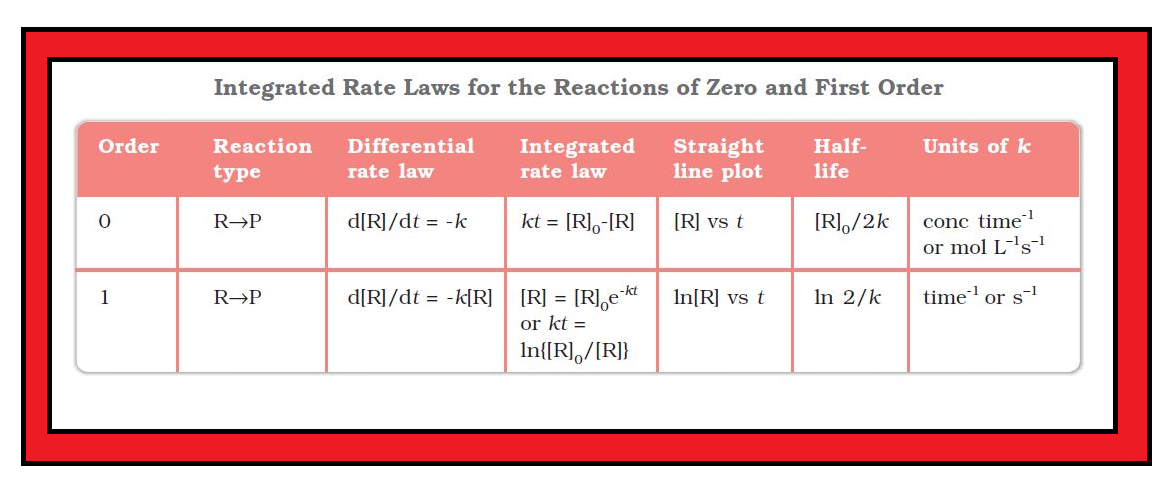
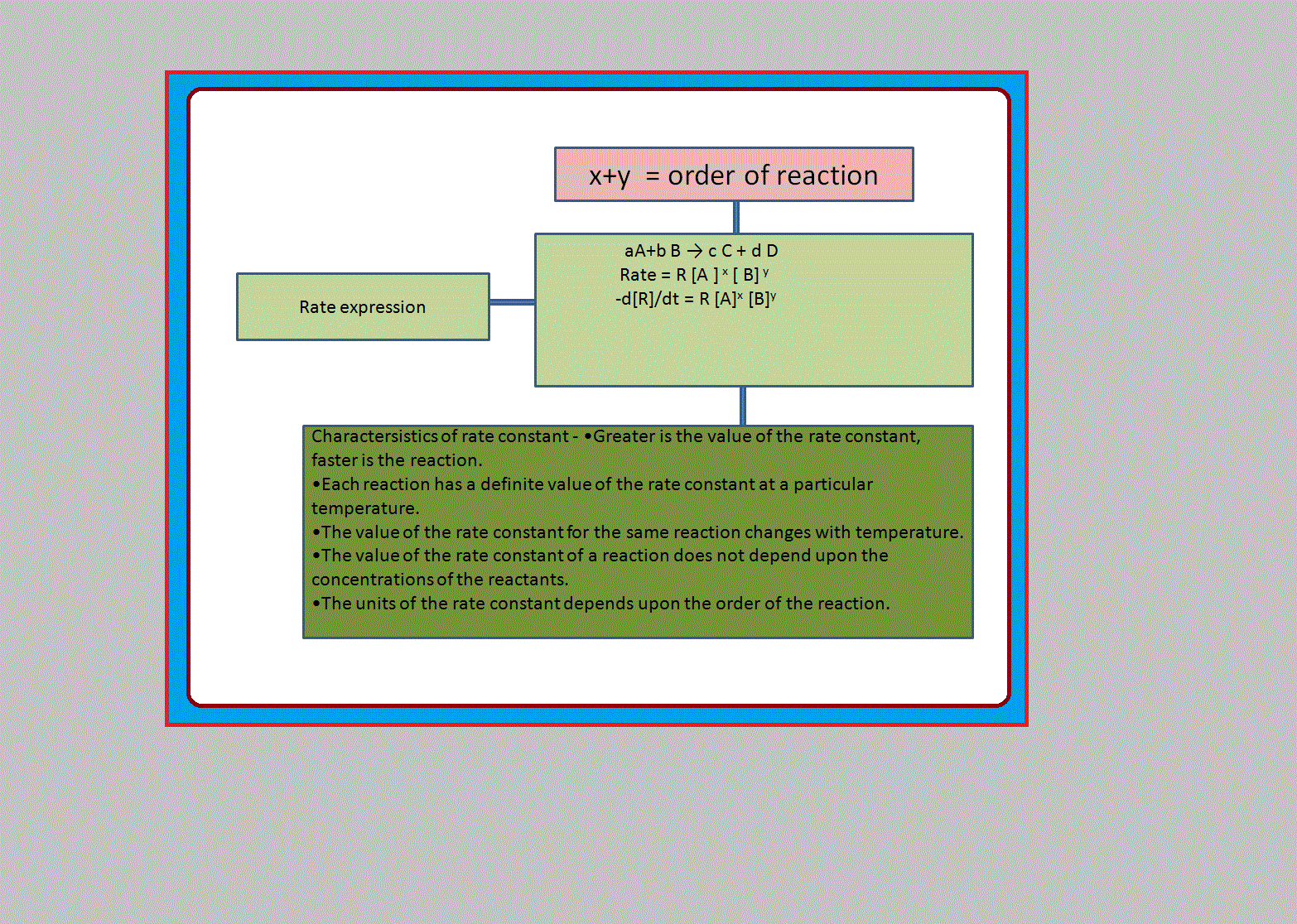
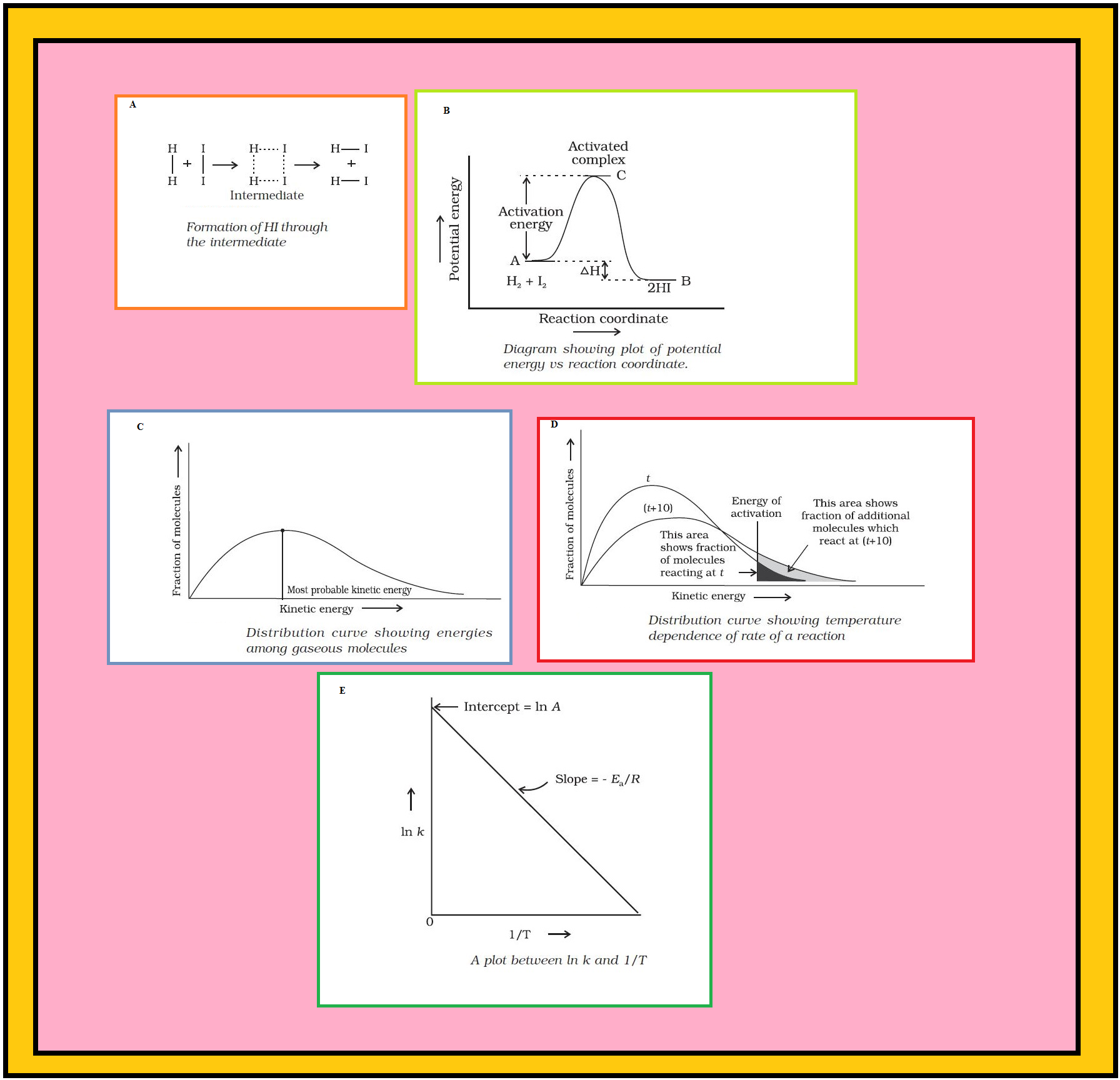
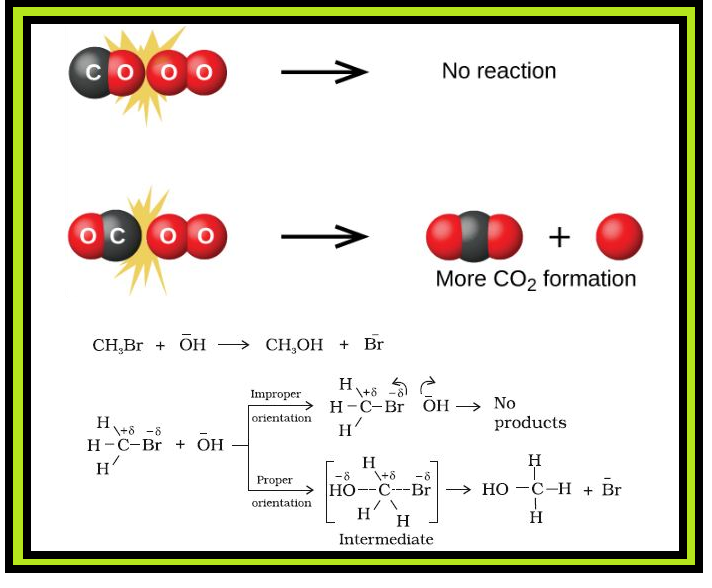
•`color{green} ("Chemical kinetics")` :The branch of chemistry which deals with the study of the speeds or the rates of chemical reactions, the factors affecting the rates of the reactions and the mechanism by which the reactions proceed is known as chemical kinetics.