Gas Laws
Gaseous state is the simple state of the matter. The behaviour of gases is governed by some general laws known as Gas laws. These laws are relationships between temperature, pressure, volume and mass.
Gas laws are as follows :
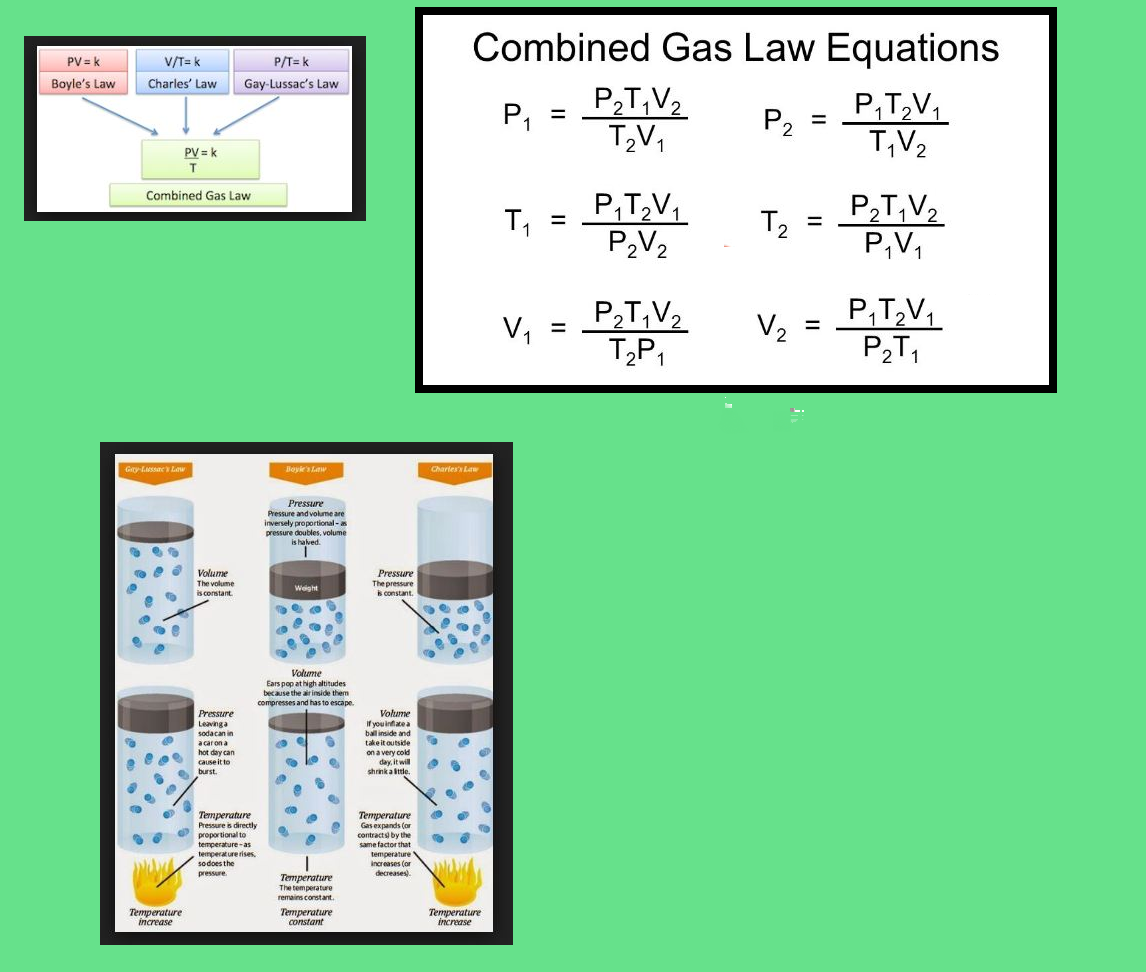
Boyle's Law According to Boyle's law, at constant temperature, pressure of a gas varies inversely with its volume.
`p prop 1/V \ \ \ \ \ \ [ " at constant T " ]`
`=> pV = K \ \ \ \ \ \ \ \ \ \ [ K = "constant" ]`
`p_1V_1 = p_2V_2`
Charles' Law According to this law, at constant pressure the volume of a given mass of a gas varies directly with
its temperature.
`V prop T \ \ \ \ \ \ \ \ \ \ \ \ \ \ [ "at constant p"]`
`V = KT \ \ \ \ \ \ \ \ \ \ \ \ [K= "constant"]`
`=> V/T = K`
`=> V_1/T_1 = V_2/T_2`
Bursting of hydrogen balloon and making of chappati are the applications of Charles' law.
Gay-Lussac's Law Aco)rding to this law, "At a constant volume, the pressure of a given mass of a gas is directly proportional to its absolute temperature."
`p prop T` or `p/T = "constant", p_1/T_1 = p_2/T_2`
Avogadro's Law Under similar conditions of temperature and pressure, equal Volume of all gases contain equal number of molecules .
or
At a given temperature and pressure, the volume of any gas is directly proportional to the number of moles of gas.
`V prop n` where, `n =` number of moles
`V/n = K "(constant)"`
Gas laws are as follows :
Boyle's Law According to Boyle's law, at constant temperature, pressure of a gas varies inversely with its volume.
`p prop 1/V \ \ \ \ \ \ [ " at constant T " ]`
`=> pV = K \ \ \ \ \ \ \ \ \ \ [ K = "constant" ]`
`p_1V_1 = p_2V_2`
Charles' Law According to this law, at constant pressure the volume of a given mass of a gas varies directly with
its temperature.
`V prop T \ \ \ \ \ \ \ \ \ \ \ \ \ \ [ "at constant p"]`
`V = KT \ \ \ \ \ \ \ \ \ \ \ \ [K= "constant"]`
`=> V/T = K`
`=> V_1/T_1 = V_2/T_2`
Bursting of hydrogen balloon and making of chappati are the applications of Charles' law.
Gay-Lussac's Law Aco)rding to this law, "At a constant volume, the pressure of a given mass of a gas is directly proportional to its absolute temperature."
`p prop T` or `p/T = "constant", p_1/T_1 = p_2/T_2`
Avogadro's Law Under similar conditions of temperature and pressure, equal Volume of all gases contain equal number of molecules .
or
At a given temperature and pressure, the volume of any gas is directly proportional to the number of moles of gas.
`V prop n` where, `n =` number of moles
`V/n = K "(constant)"`
