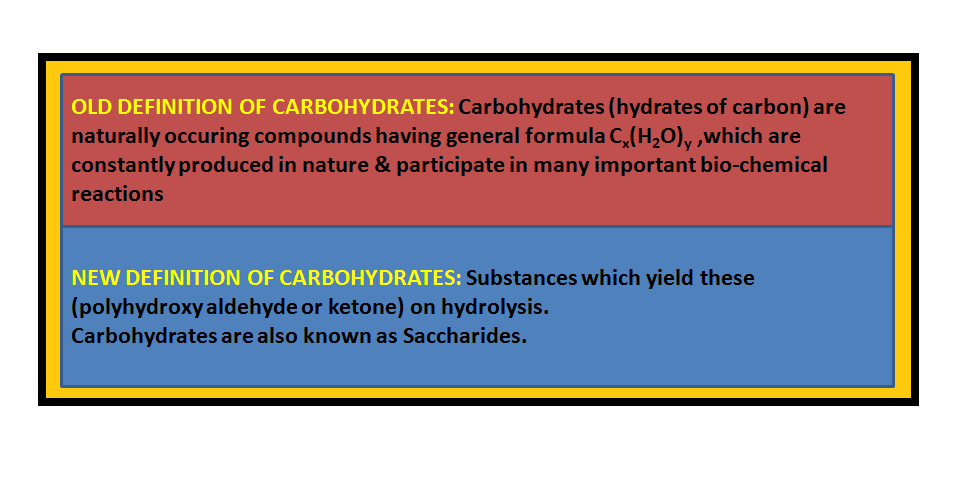
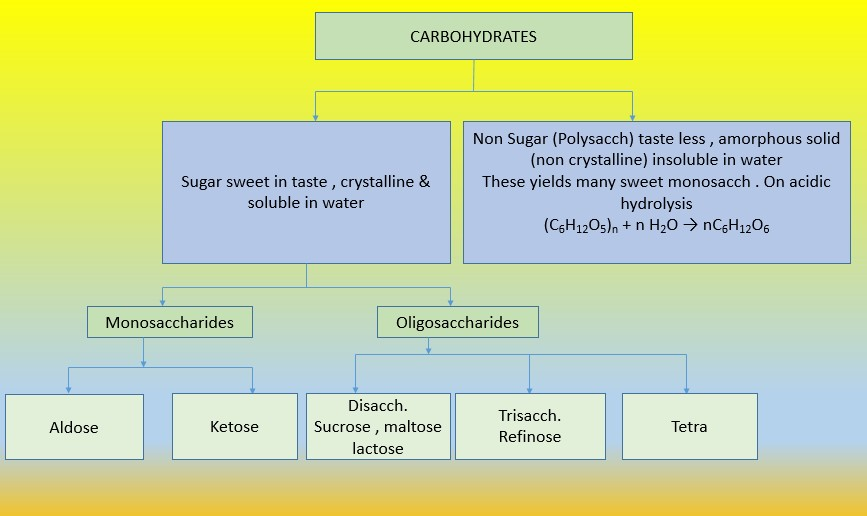
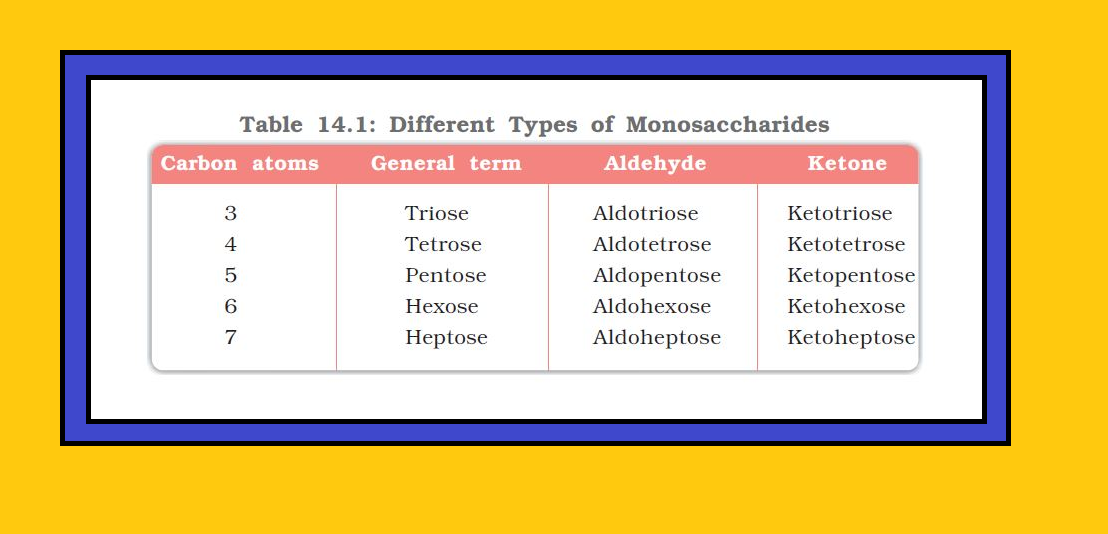
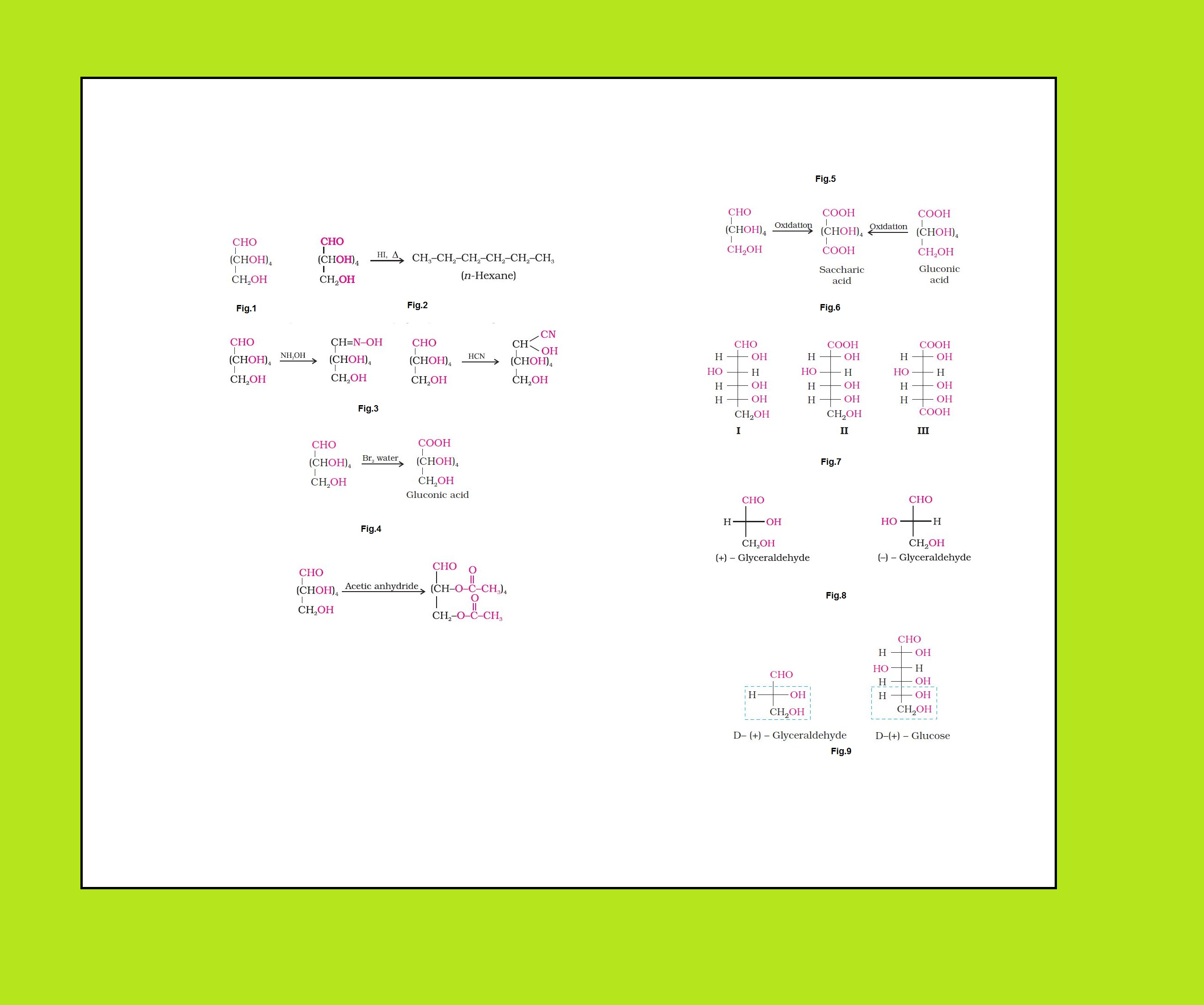
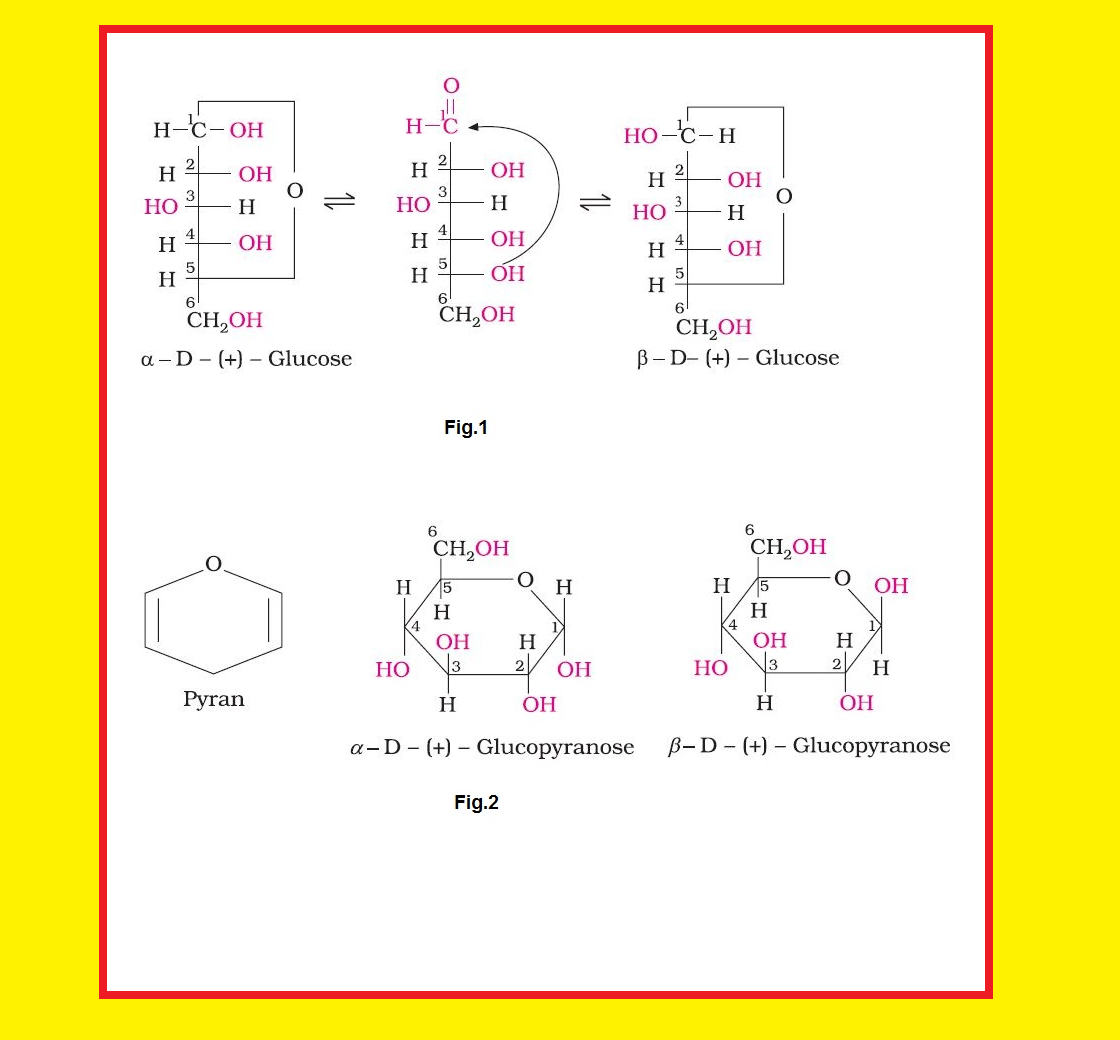
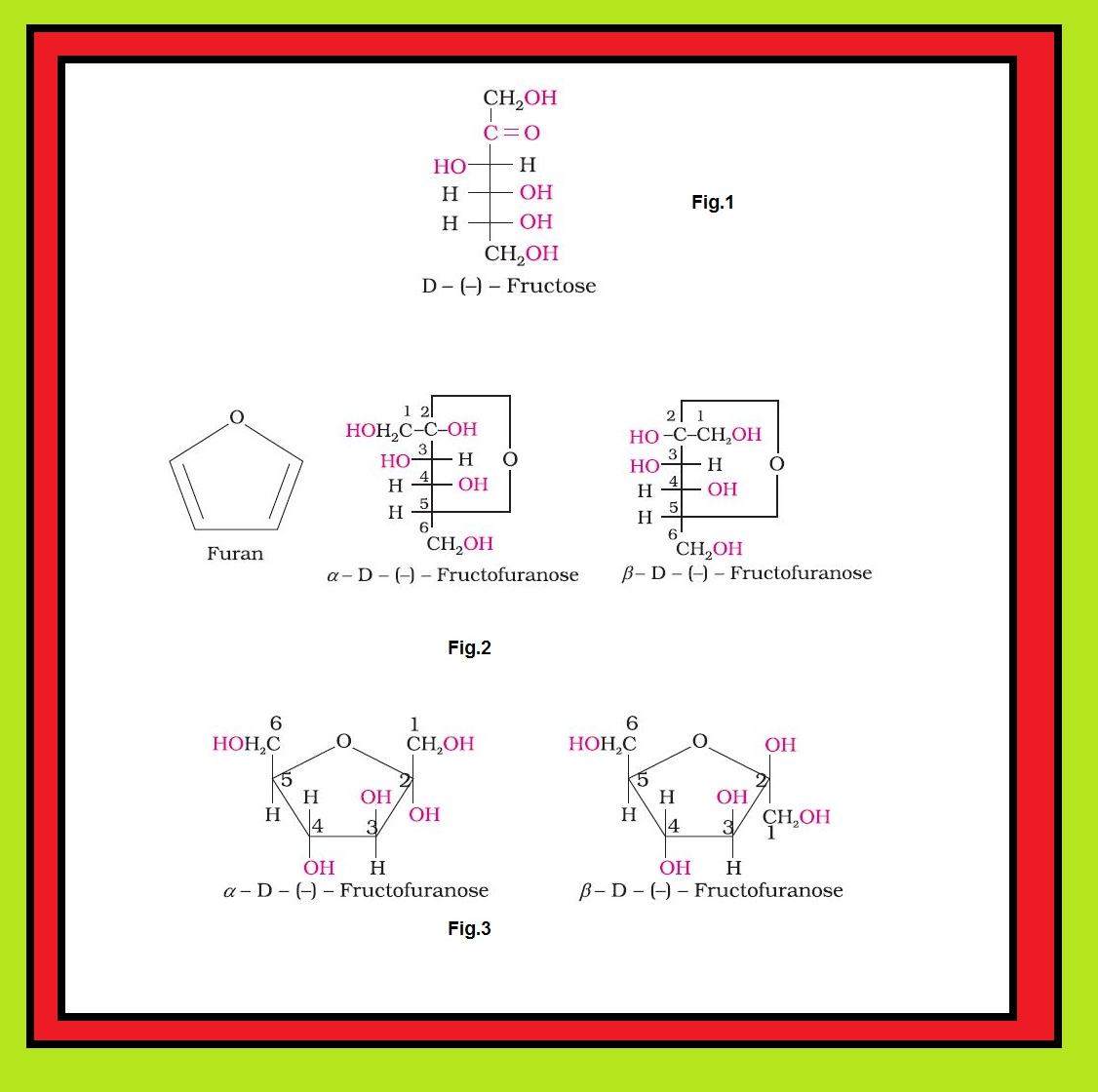
• `color{green}("Monosaccharides ")` : A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide. About `20` monosaccharides are known to occur in nature. Some common examples are glucose, fructose, ribose, etc.
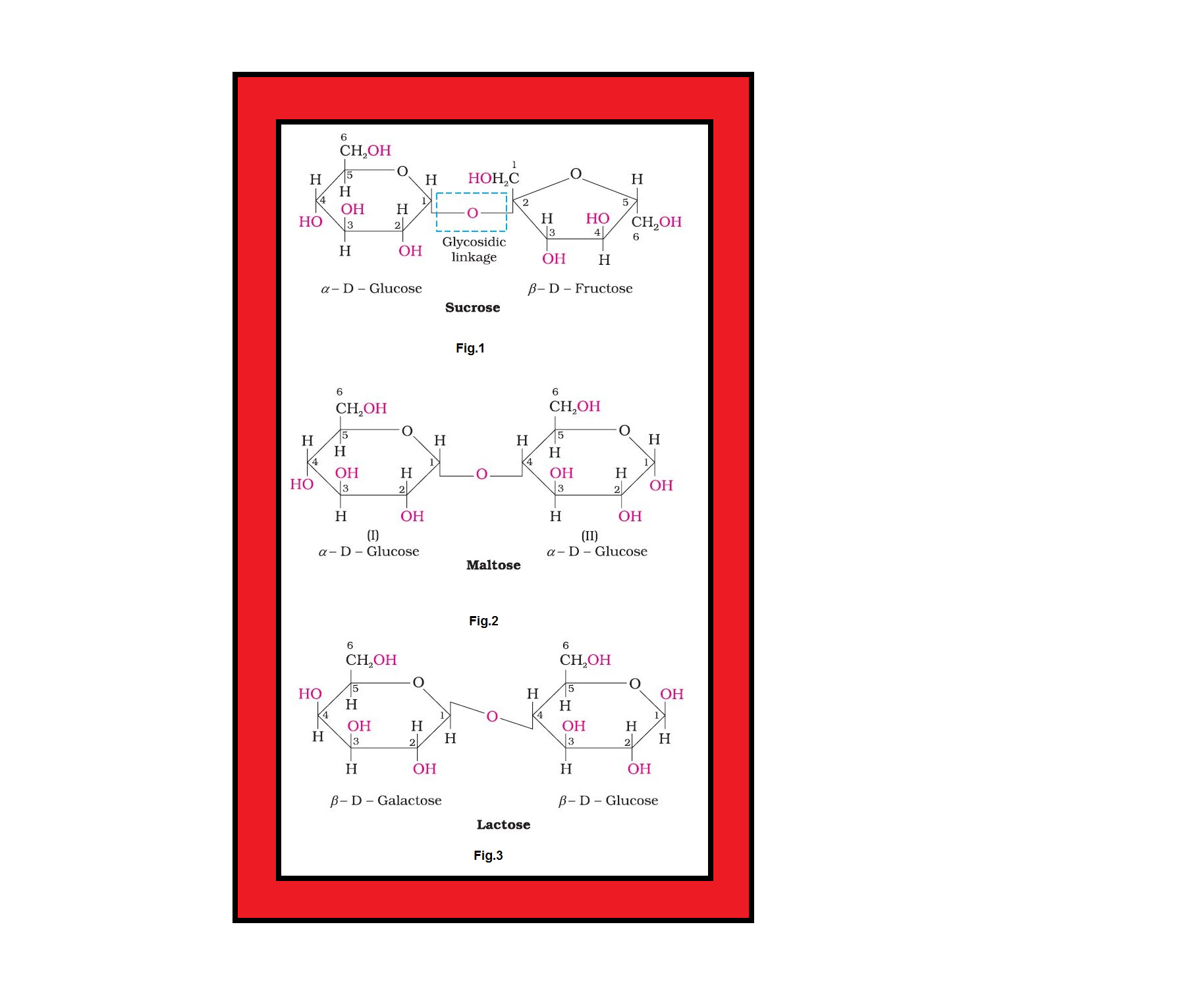
• `color{green}("Oligosaccharides ")` : Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides.
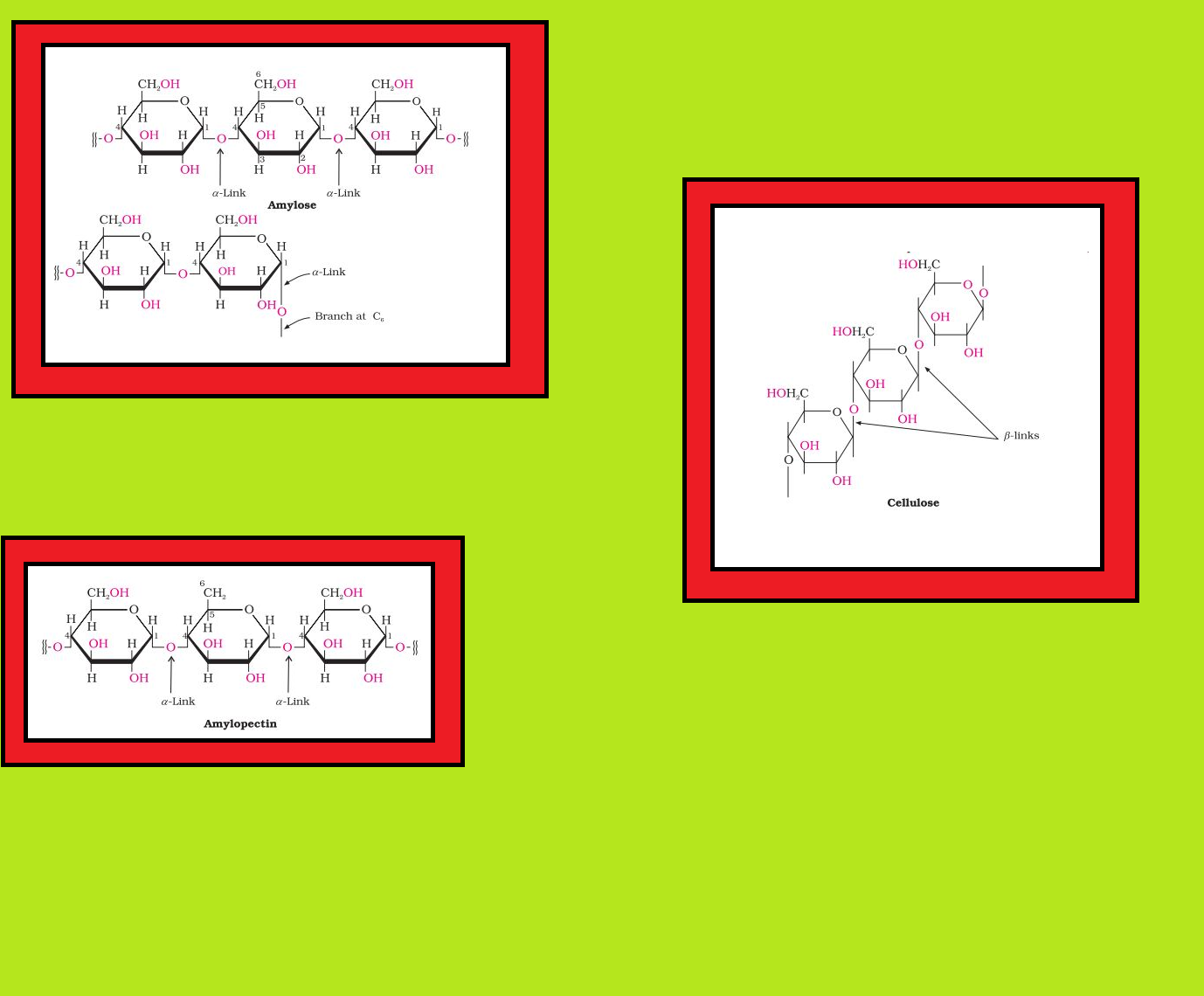
• `color{green}("Polysaccharides ")` : Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides.
• `color{green}("Non-reducing sugars ")` : In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars e.g. sucrose.
• `color{green}("Reducing sugars ")` :On the other hand, sugars in which these functional groups are free, are called reducing sugars, for example, maltose and lactose.
• `color{green}("Monosaccharides ")` : A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone is called a monosaccharide. About `20` monosaccharides are known to occur in nature. Some common examples are glucose, fructose, ribose, etc.
• `color{green}("Oligosaccharides ")` : Carbohydrates that yield two to ten monosaccharide units, on hydrolysis, are called oligosaccharides.
• `color{green}("Polysaccharides ")` : Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides.
• `color{green}("Non-reducing sugars ")` : In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars e.g. sucrose.
• `color{green}("Reducing sugars ")` :On the other hand, sugars in which these functional groups are free, are called reducing sugars, for example, maltose and lactose.