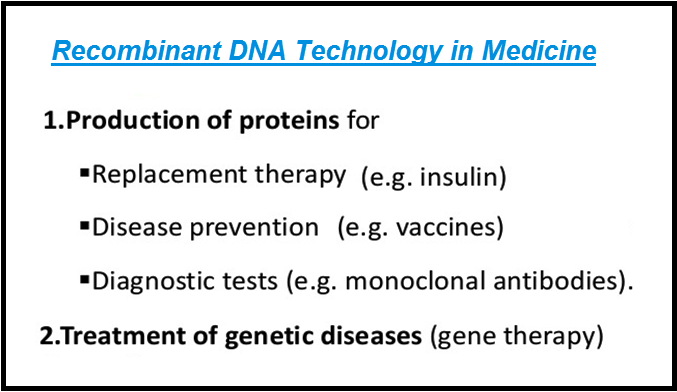
● Management of `color{violet}("adult-onset diabetes")` is possible by taking `color{violet}("insulin")` at `color{violet}("regular time")` intervals.
● `color{violet}("Insulin")` used for `color{violet}("diabetes")` was earlier extracted from `color{violet}("pancreas")` of `color{violet}("slaughtered cattle")` and `color{violet}("pigs")`.
● `color{violet}("Insulin")` from an `color{violet}("animal source")`, though caused some patients to develop allergy or other types of reactions to the `color{violet}("foreign protein")`.
● `color{violet}("Insulin")` consists of two `color{violet}("short polypeptide chains: chain A and chain B")`, that are linked together by `color{violet}("disulphide bridges")`.

● In `color{violet}("mammals, including humans, insulin")` is `color{violet}("synthesised")` as a `color{violet}("prohormone")` (like a pro-enzyme, the pro-hormone also needs to be processed before it becomes a fully mature and functional hormone) which contains an extra stretch called the `color{violet}("C peptide")`.
● This `color{violet}("C peptide")` is not present in the `color{violet}("mature insulin")` and is removed during `color{violet}("maturation")` into `color{violet}("insulin")`.
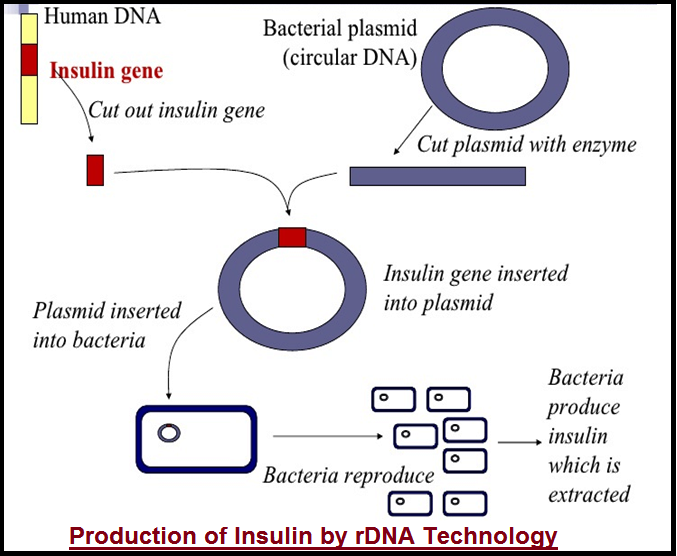
● The main challenge for `color{violet}("production of insulin")` using `color{violet}("rDNA")` techniques was getting `color{violet}("insulin assembled")` into a `color{violet}("mature form")`.
● In `color{violet}("1983, Eli Lilly")` an American company prepared two `color{violet}("DNA")` sequences corresponding to A and B, chains of `color{violet}("human insulin")` and introduced them in `color{violet}("plasmids of E. coli")` to produce`color{violet}(" insulin chains")`.
● `color{violet}("Chains A and B")` were produced separately, extracted and combined by `color{violet}("creating disulfide bonds")` to form `color{violet}("human insulin")`.
● Management of `color{violet}("adult-onset diabetes")` is possible by taking `color{violet}("insulin")` at `color{violet}("regular time")` intervals.
● `color{violet}("Insulin")` used for `color{violet}("diabetes")` was earlier extracted from `color{violet}("pancreas")` of `color{violet}("slaughtered cattle")` and `color{violet}("pigs")`.
● `color{violet}("Insulin")` from an `color{violet}("animal source")`, though caused some patients to develop allergy or other types of reactions to the `color{violet}("foreign protein")`.
● `color{violet}("Insulin")` consists of two `color{violet}("short polypeptide chains: chain A and chain B")`, that are linked together by `color{violet}("disulphide bridges")`.

● In `color{violet}("mammals, including humans, insulin")` is `color{violet}("synthesised")` as a `color{violet}("prohormone")` (like a pro-enzyme, the pro-hormone also needs to be processed before it becomes a fully mature and functional hormone) which contains an extra stretch called the `color{violet}("C peptide")`.
● This `color{violet}("C peptide")` is not present in the `color{violet}("mature insulin")` and is removed during `color{violet}("maturation")` into `color{violet}("insulin")`.
● The main challenge for `color{violet}("production of insulin")` using `color{violet}("rDNA")` techniques was getting `color{violet}("insulin assembled")` into a `color{violet}("mature form")`.
● In `color{violet}("1983, Eli Lilly")` an American company prepared two `color{violet}("DNA")` sequences corresponding to A and B, chains of `color{violet}("human insulin")` and introduced them in `color{violet}("plasmids of E. coli")` to produce`color{violet}(" insulin chains")`.
● `color{violet}("Chains A and B")` were produced separately, extracted and combined by `color{violet}("creating disulfide bonds")` to form `color{violet}("human insulin")`.