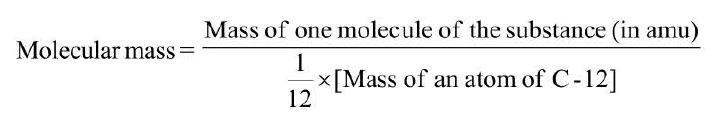Atomic mass
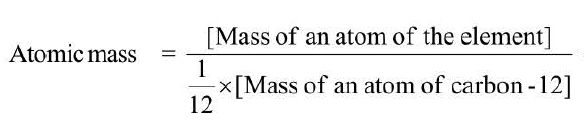
Atomic mass of an element is the number which indicates how many times the mass of one atom of the element is heavier in comparison to `1/12`th part of the mass of one atom of Carbon- 12. It is expressed in units of `am``u`.
1 amu =` 1.66 xx 10^-27` kg = `1.66 xx 10^-24` gm = `1/N_A` gm
Funda: Gram atomic mass is the mass of 1 mol atom of an element. Its unit is `gram`.
Conceptual Illustration:-
(a) What is the mass of one atom of `Cl`?
(b) What is the atomic mass of `Cl`?
(c) What is the gram atomic mass of `Cl`?
Sol. (a) Mass of one atom of Cl = `35.5` amu.
(b) Atomic mass of Cl = Mass of an atom in amu/ 1 amu
= `35.5` amu/1 amu =`35.5`
(c) Gram atomic massof Cl = Mass of one `Cl` atom x `N_A`
= `35.5` amu x `N_A`
= `35.5/N_A`x `N_A` gram = `35.5` gram
1 amu =` 1.66 xx 10^-27` kg = `1.66 xx 10^-24` gm = `1/N_A` gm
Funda: Gram atomic mass is the mass of 1 mol atom of an element. Its unit is `gram`.
Conceptual Illustration:-
(a) What is the mass of one atom of `Cl`?
(b) What is the atomic mass of `Cl`?
(c) What is the gram atomic mass of `Cl`?
Sol. (a) Mass of one atom of Cl = `35.5` amu.
(b) Atomic mass of Cl = Mass of an atom in amu/ 1 amu
= `35.5` amu/1 amu =`35.5`
(c) Gram atomic massof Cl = Mass of one `Cl` atom x `N_A`
= `35.5` amu x `N_A`
= `35.5/N_A`x `N_A` gram = `35.5` gram