Thomson's Model :

The plum pudding model is one of several scientific models of the atom. First proposed by J. J. Thomson in 1904 soon after the discovery of the electron, but before the discovery of the atomic nucleus, the model represented an attempt to consolidate the known properties of atoms at the time: 1) electrons are negatively-charged particles and 2) atoms are neutrally-charged.
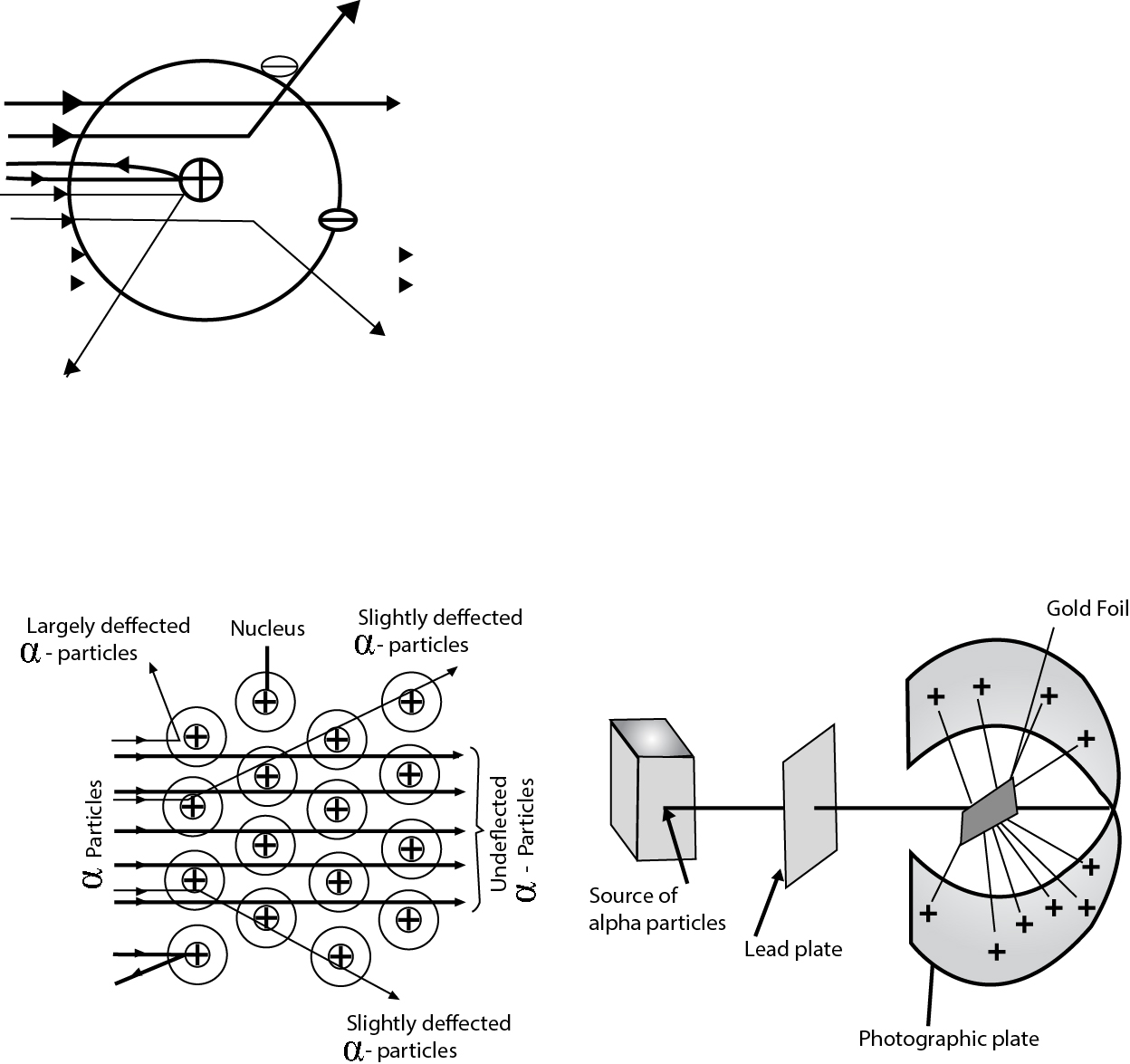
J J Thomson, in 1904, proposed that there was an equal and opposite positive charge enveloping the electrons in a matrix. This model is called the plum- pudding model after a type of Victorian dessert in which bits of plums were surrounded by matrix of pudding
This model could not satisfactorily explain the results of scattering experiment carried out by Rutherford who worked with Thomson.
J J Thomson, in 1904, proposed that there was an equal and opposite positive charge enveloping the electrons in a matrix. This model is called the plum- pudding model after a type of Victorian dessert in which bits of plums were surrounded by matrix of pudding
This model could not satisfactorily explain the results of scattering experiment carried out by Rutherford who worked with Thomson.