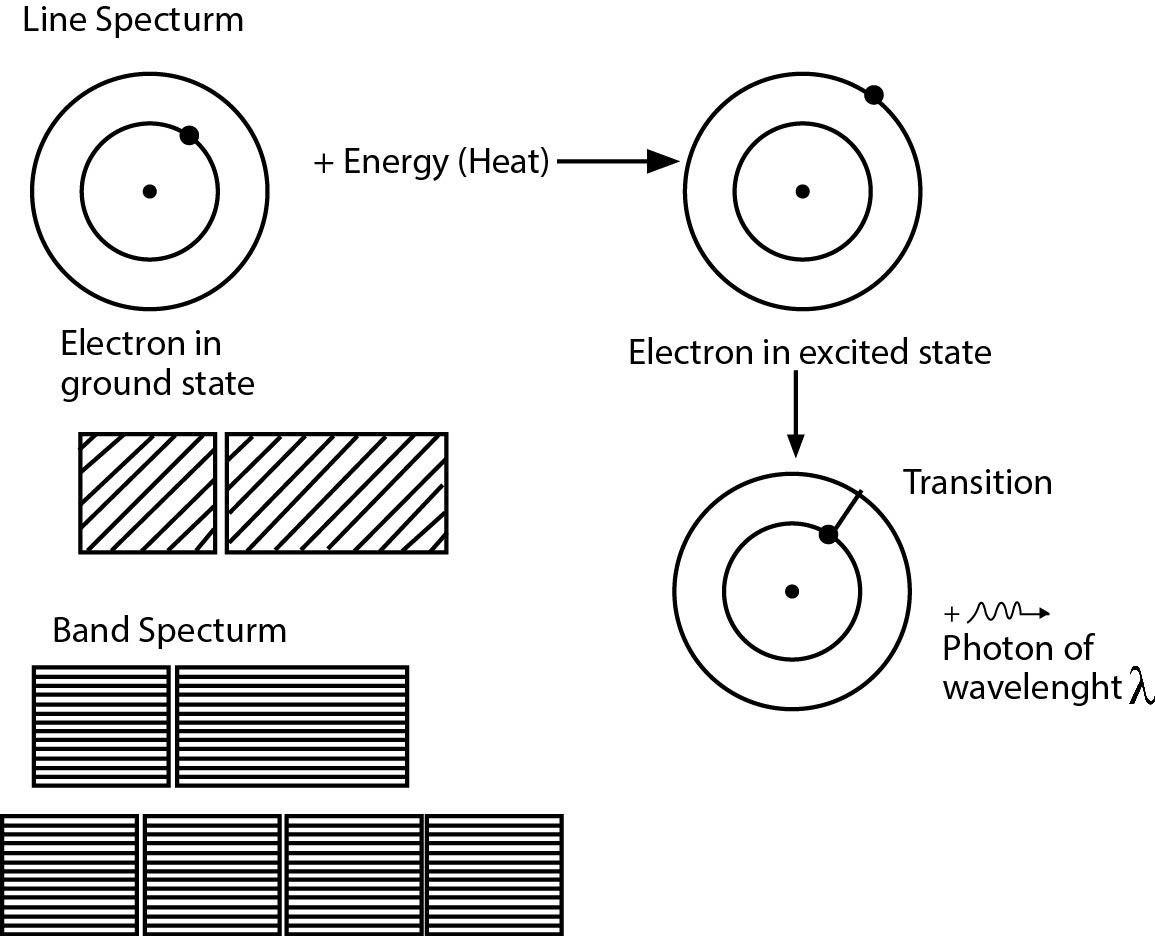
Spectrum :

When light coming from a source is dispersed by a prism, light of different wavelength are deviated through different angles and get separated. This phenomenon is called dispersion and such a dispersed light may be received on a photographic plate or it may be viewed directly by eye. A collection of dispersed light giving its wave length composition is called a spectrum.