Volume Correction :
`V` in the ideal gas equation represents the volume where the molecules can move freely. In real gases, a part of the total volume is, however, occupied by the molecules of the gas . If `b` represents the effective volume occupied by the molecules of `1` mole of a gas, then for the amount `n` moles of the gas `V`; is given by `V = V_(text(container)) -nb`
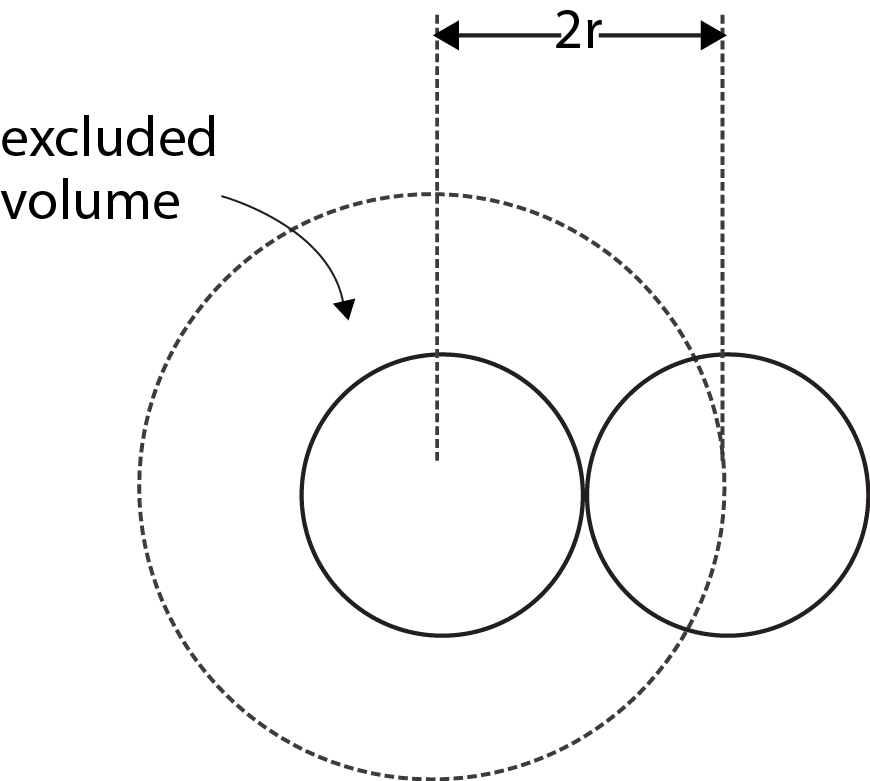
where `b` is called the excluded volume or co-volume. The numerical value of `b` is four times the actual volume occupied by the gas molecules. This can be shown as follows. If we consider only bimolecular collisions, then the volume occupied by the sphere of radius `2r` represents the excluded volume per pair of molecules as shown in Fig
Thus excluded volume per pair of molecules
`= 4/3 pi (2r)^3=8(4/3 pi r^3)`
Excluded volume per molecule
`=1/2[8(4/3 pi r^3)] -4(4/3 pi r^3) = 4xx` (volume occupied by a molecule)
Since `b` represents excluded volume per mole of the gas, it is obvious that `b= N_A[4(4/3 pi r^3)]`
where `b` is called the excluded volume or co-volume. The numerical value of `b` is four times the actual volume occupied by the gas molecules. This can be shown as follows. If we consider only bimolecular collisions, then the volume occupied by the sphere of radius `2r` represents the excluded volume per pair of molecules as shown in Fig
Thus excluded volume per pair of molecules
`= 4/3 pi (2r)^3=8(4/3 pi r^3)`
Excluded volume per molecule
`=1/2[8(4/3 pi r^3)] -4(4/3 pi r^3) = 4xx` (volume occupied by a molecule)
Since `b` represents excluded volume per mole of the gas, it is obvious that `b= N_A[4(4/3 pi r^3)]`