Compressibility Factor :
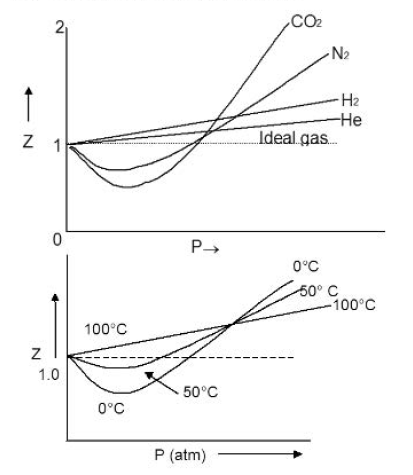
The deviations from ideal behavior can be displayed more clearly, by plotting the ratio of the observed molar volume `V_m` to the ideal molar volume `V_(m,text(ideal))` (=`(RT)//p`) as a function of pressure at constant temperature. This ratio is called the compressibility
factor `Z` and can be expressed as
`Z = V_m/V_(m,text(ideal))` = `p/(RT) V_m`
For an ideal gas `Z=1` and is independent of pressure and temperature. For a real gas, `Z = (T, p)`, is a function of both temperature and
pressure.
i) At low pressures: `V` is large and `b` is negligible in comparison with `V`. The Van der Waals equation reduces to:
`(p + a/V^2)V = RT`; `pV + a/V= RT`
`pV = RT - a/V` or `pV < RT`
This accounts for the dip in `pV` vs `p` isotherm at low pressures.
ii) At fairly high pressures:
a/V^2 may be neglected in comparison with `p`. The Van der Waals equation becomes
`p (V- b) = RT`
`pV-pb = RT`
iii) At very low pressures:
`V` becomes so large that both `b` and `a/V^2` become negligible and the Van der Waals equation reduces to `pV = RT.` This shows why gases approach ideal behaviour at very low pressures.
iv) Hydrogen and Helium:
These are two lightest gases known. Their molecules have very small masses. The attractive forces between such molecules will be extensively small. So, `a/V^2` is negligible even at ordinary temperatures. Thus `pV > RT`.
factor `Z` and can be expressed as
`Z = V_m/V_(m,text(ideal))` = `p/(RT) V_m`
For an ideal gas `Z=1` and is independent of pressure and temperature. For a real gas, `Z = (T, p)`, is a function of both temperature and
pressure.
i) At low pressures: `V` is large and `b` is negligible in comparison with `V`. The Van der Waals equation reduces to:
`(p + a/V^2)V = RT`; `pV + a/V= RT`
`pV = RT - a/V` or `pV < RT`
This accounts for the dip in `pV` vs `p` isotherm at low pressures.
ii) At fairly high pressures:
a/V^2 may be neglected in comparison with `p`. The Van der Waals equation becomes
`p (V- b) = RT`
`pV-pb = RT`
iii) At very low pressures:
`V` becomes so large that both `b` and `a/V^2` become negligible and the Van der Waals equation reduces to `pV = RT.` This shows why gases approach ideal behaviour at very low pressures.
iv) Hydrogen and Helium:
These are two lightest gases known. Their molecules have very small masses. The attractive forces between such molecules will be extensively small. So, `a/V^2` is negligible even at ordinary temperatures. Thus `pV > RT`.