Double Salts and Co-ordination Compound :
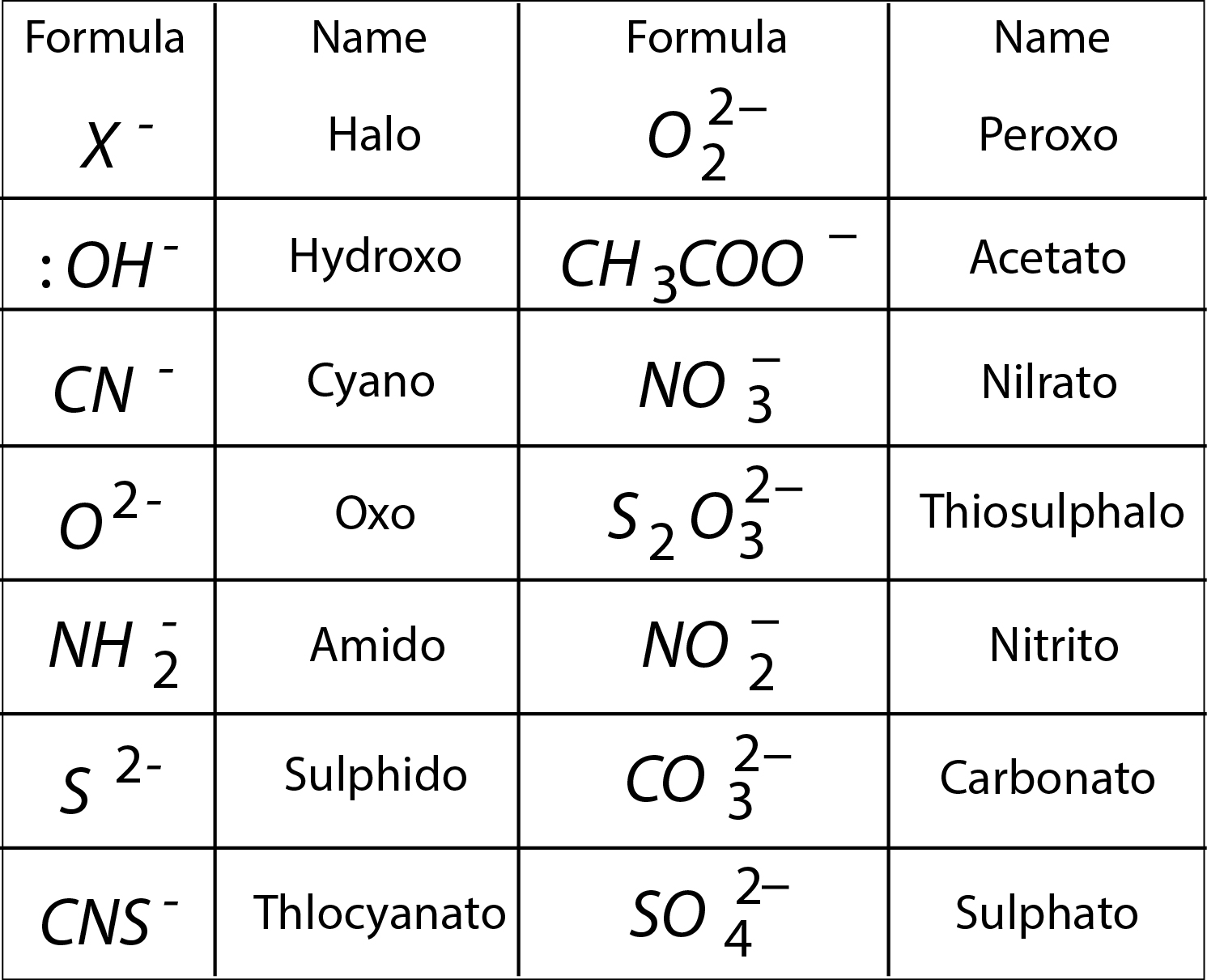
When solutions of two or more stable compounds are mixed in stoichiometric (simple molecular) proportions new crystalline compounds called molecular or addition compounds are formed. These are of two types :
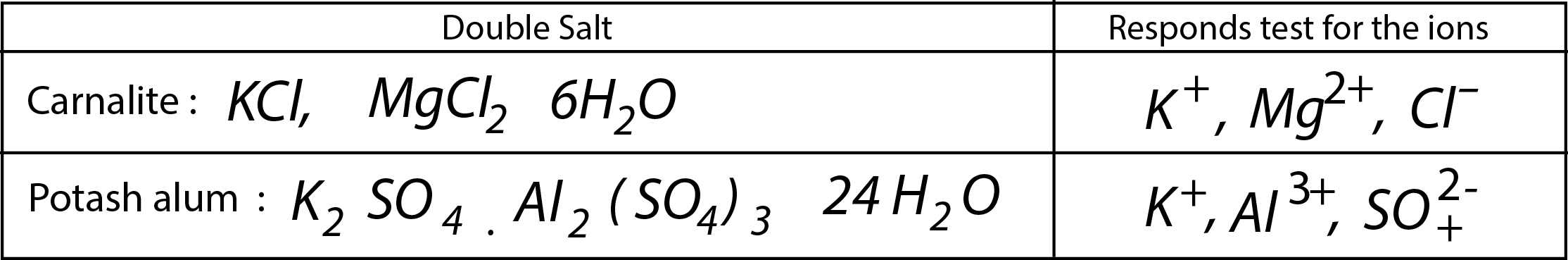
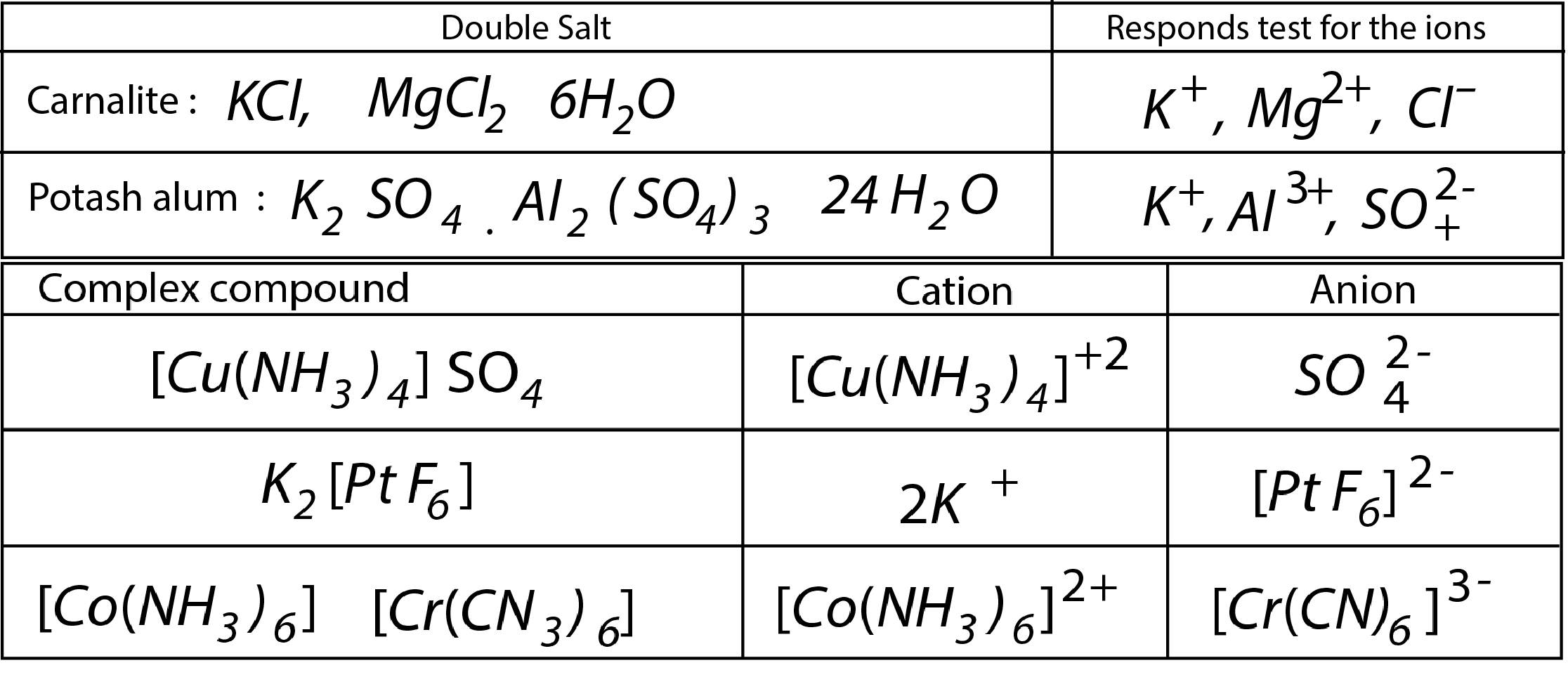
(a) `text(Double Salts)` : Addition compounds stable in solid state. Dissociate into ions in aqueous solution as such give test for each constituent ion. Examples:
(b) `text(Co-ordination or Complex compounds)` : Addition compound, stable in solid state. Retain their identity even in solution. Central metal ion form dative or coordinate bond with the species surrounding it (ligands). Examples :
(a) `text(Double Salts)` : Addition compounds stable in solid state. Dissociate into ions in aqueous solution as such give test for each constituent ion. Examples:
(b) `text(Co-ordination or Complex compounds)` : Addition compound, stable in solid state. Retain their identity even in solution. Central metal ion form dative or coordinate bond with the species surrounding it (ligands). Examples :