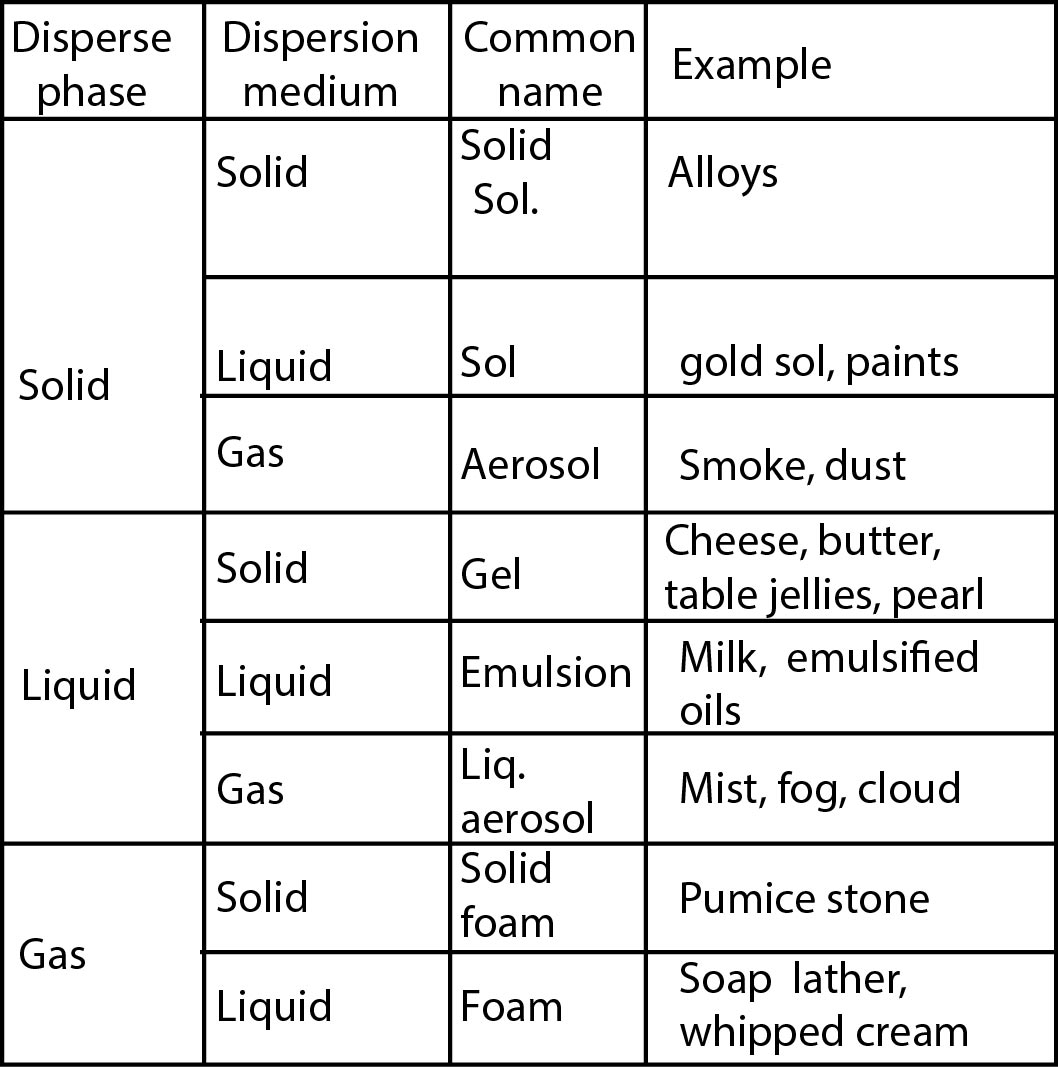
Thomas Graham tried to classify solids into (i) crystalloids and (ii) colloids based on the diffusion of dissolved solids through parchment paper or animal membrane. Substances like common salt, urea, sugar etc., which readily pass through the membrane while in the dissolved state were called crystalloids while substances like starch, gelatin, gum Arabic etc. which in the dissolved state either do not pass through the membrane or pass through very slowly were called colloids. This classification was rejected because the same substance under one set of conditions behaved like a crystalloid and under other set of conditions behaved like a colloid. For example, `NaCl` in water behaves like a crystalloid but in benzene behaves like a colloid. Soap in water behaves like a colloid while in alcohol it behaves, like a crystalloid. Therefore, there is no separate class of substances called colloidal substance. It is just a state of matter into which every substance can be obtained by a suitable method. The nature of a substance whether crystalloid or colloid depends upon the size of the solute particles. When the size of solute particles lies between `1 nm` to `100 nm` it behaves like a colloid. If size of solute particles is greater than `100 nm`, it exists as suspension and if particle size is less than `1 nm` it exists as a true solution. Colloidal solution is heterogeneous in nature and always consists of at least two phases - namely disperse phase and dispersion medium. The component present in small proportion and consisting of particles of colloidal dimensions is called disperse phase. The medium in which colloidal particles are dispersed is called dispersion medium. The two phases can be solid- liquid or -gas. There are eight different types of colloidal solutions.
If colloidal solution has fluid like appearance it is called sol. The dispersion medium in such cases is generally liquid. Depending upon the nature of medium, colloids are sometimes given special names. For example
| Dispersion medium | Name of the sol |
| Water | Hydrosol |
| Alcohol | Alcosol |
| Benzene | Benzosol |
| Gases | Aerosol |
Thomas Graham tried to classify solids into (i) crystalloids and (ii) colloids based on the diffusion of dissolved solids through parchment paper or animal membrane. Substances like common salt, urea, sugar etc., which readily pass through the membrane while in the dissolved state were called crystalloids while substances like starch, gelatin, gum Arabic etc. which in the dissolved state either do not pass through the membrane or pass through very slowly were called colloids. This classification was rejected because the same substance under one set of conditions behaved like a crystalloid and under other set of conditions behaved like a colloid. For example, `NaCl` in water behaves like a crystalloid but in benzene behaves like a colloid. Soap in water behaves like a colloid while in alcohol it behaves, like a crystalloid. Therefore, there is no separate class of substances called colloidal substance. It is just a state of matter into which every substance can be obtained by a suitable method. The nature of a substance whether crystalloid or colloid depends upon the size of the solute particles. When the size of solute particles lies between `1 nm` to `100 nm` it behaves like a colloid. If size of solute particles is greater than `100 nm`, it exists as suspension and if particle size is less than `1 nm` it exists as a true solution. Colloidal solution is heterogeneous in nature and always consists of at least two phases - namely disperse phase and dispersion medium. The component present in small proportion and consisting of particles of colloidal dimensions is called disperse phase. The medium in which colloidal particles are dispersed is called dispersion medium. The two phases can be solid- liquid or -gas. There are eight different types of colloidal solutions.
If colloidal solution has fluid like appearance it is called sol. The dispersion medium in such cases is generally liquid. Depending upon the nature of medium, colloids are sometimes given special names. For example
| Dispersion medium | Name of the sol |
| Water | Hydrosol |
| Alcohol | Alcosol |
| Benzene | Benzosol |
| Gases | Aerosol |