Alkanes :
Alkanes (Paraffin): The compounds of carbon and hydrogen with the general formula `C_n H_(2n+2)` are called as alkanes. These are also known as saturated hydrocarbons or Paraffins.
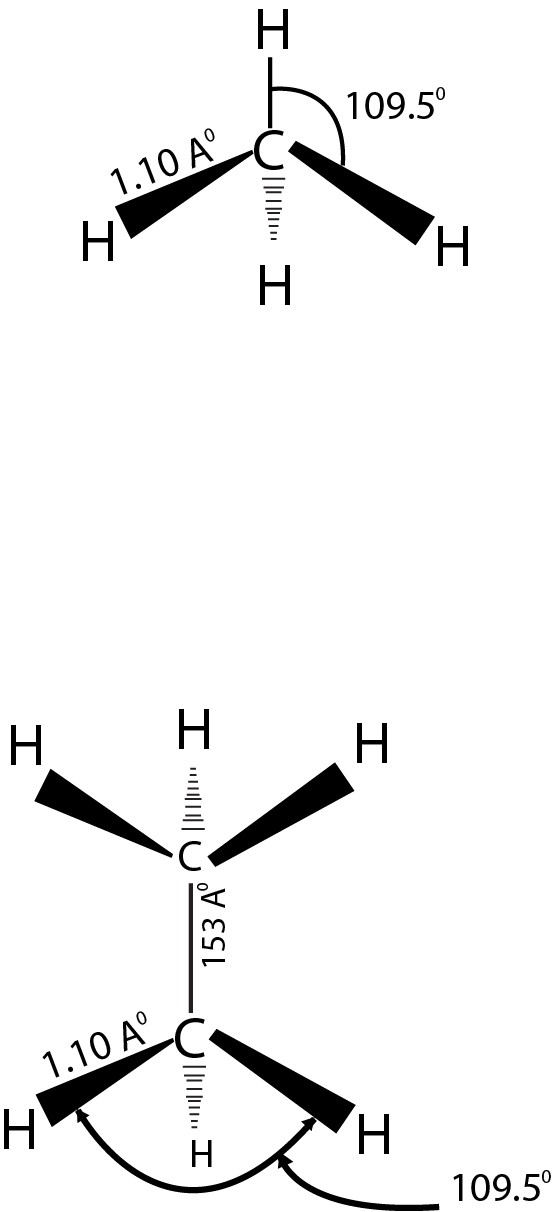
Structure and Reactivity: The simplest member of this fami ly is methane `(CH_4)` where the `C`-atom is `sp^3` hybridized, which are overlapping with the `s-`orbitals of `H-`atoms at the corner of regular tetrahedron.This structure is verified by electron
diffraction method.
The next higher member in the family is ethane `(C_2 H_6)`.
Here again each `C`-atom is `sp ^3`-hybridized with a bond angle of approximately `109.5^(o)`
If one consider a molecule of methane or ethane or any other alkane, we find that all these molecules are non polar and hence the operative interactive forces are Vander Waal's forces.
Structure and Reactivity: The simplest member of this fami ly is methane `(CH_4)` where the `C`-atom is `sp^3` hybridized, which are overlapping with the `s-`orbitals of `H-`atoms at the corner of regular tetrahedron.This structure is verified by electron
diffraction method.
The next higher member in the family is ethane `(C_2 H_6)`.
Here again each `C`-atom is `sp ^3`-hybridized with a bond angle of approximately `109.5^(o)`
If one consider a molecule of methane or ethane or any other alkane, we find that all these molecules are non polar and hence the operative interactive forces are Vander Waal's forces.