Gas laws:-
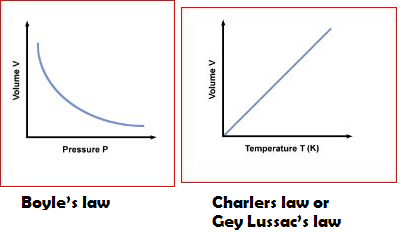
(a) Boyle's law:- It states that the volume of a given amount of gas varies inversely as its pressure, provided its temperature is kept constant.
PV = Constant
(b)Charlers law or Gey Lussac's law:- It states that volume of a given mass of a gas varies directly as its absolute temperature, provided its pressure is kept constant.
`V/T` = constant
(c)Gay Lussac's law of pressure:- It states that pressure of a given mass of a gas varies directly as its absolute temperature provided the volume of the gas is kept constant.
`P/T = P_0/T_0 or P = P_0/T_0 T = 1/273 = γp`
(d) Dalton's law of partial pressures:-
Partial pressure of a gas or of saturated vapors is the pressure which it would exert if contained alone in the entire confined given space.
`P= p_1+p_2+p_3+....`
(e) Avogadro's law:- It states that under similar conditions of pressure and temperature, equal volume of all gases contain equal number of molecules.
For m gram of gas, `PV/T = nR = (m/M) R`
PV = Constant
(b)Charlers law or Gey Lussac's law:- It states that volume of a given mass of a gas varies directly as its absolute temperature, provided its pressure is kept constant.
`V/T` = constant
(c)Gay Lussac's law of pressure:- It states that pressure of a given mass of a gas varies directly as its absolute temperature provided the volume of the gas is kept constant.
`P/T = P_0/T_0 or P = P_0/T_0 T = 1/273 = γp`
(d) Dalton's law of partial pressures:-
Partial pressure of a gas or of saturated vapors is the pressure which it would exert if contained alone in the entire confined given space.
`P= p_1+p_2+p_3+....`
(e) Avogadro's law:- It states that under similar conditions of pressure and temperature, equal volume of all gases contain equal number of molecules.
For m gram of gas, `PV/T = nR = (m/M) R`