Introduction
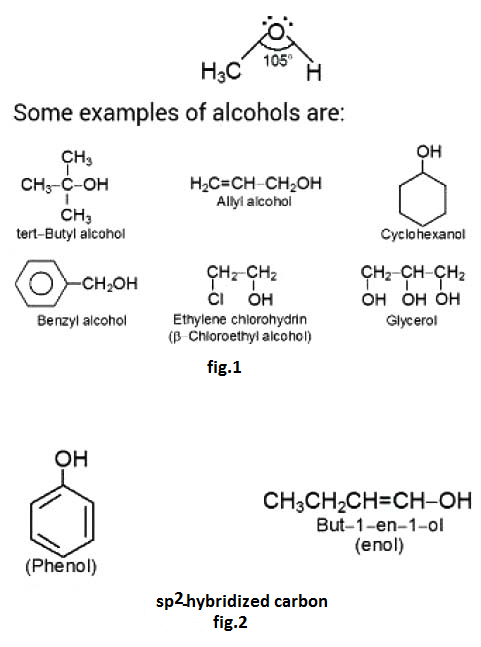
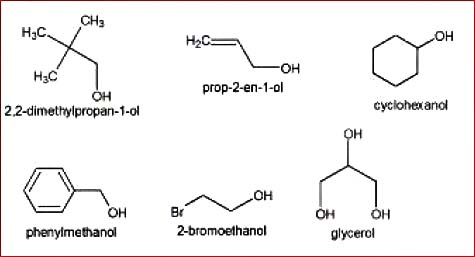
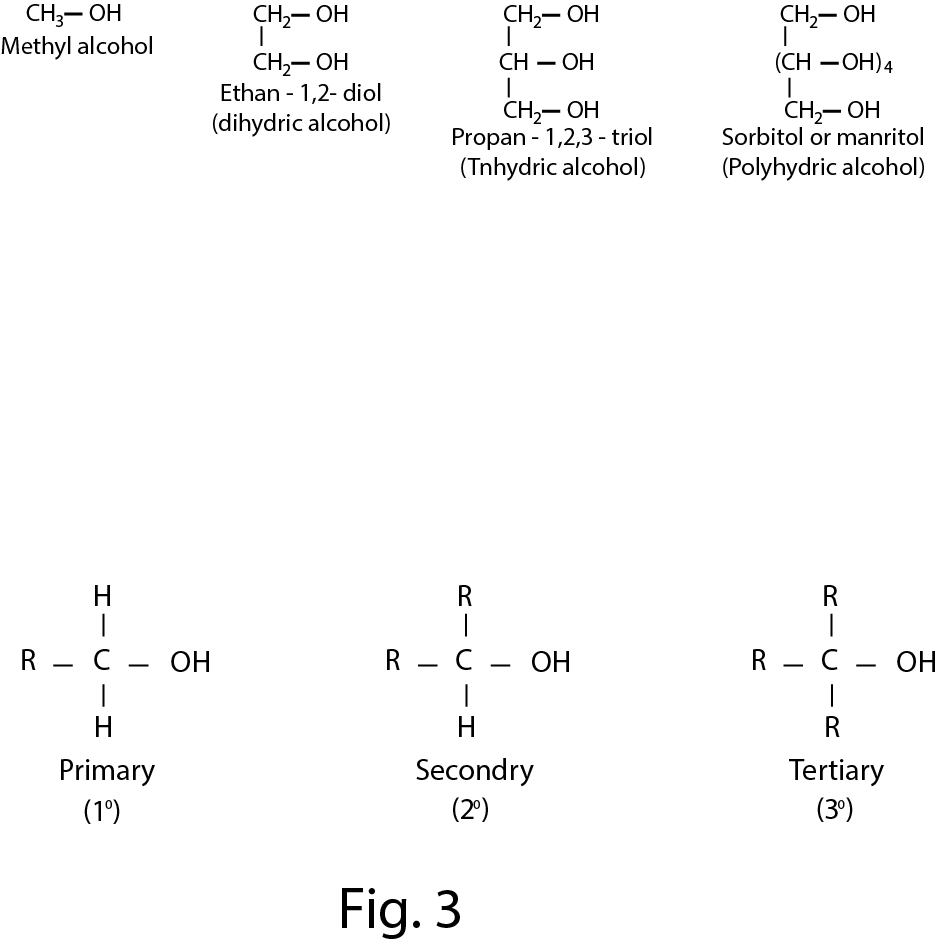
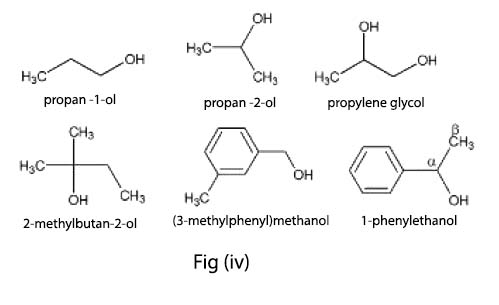
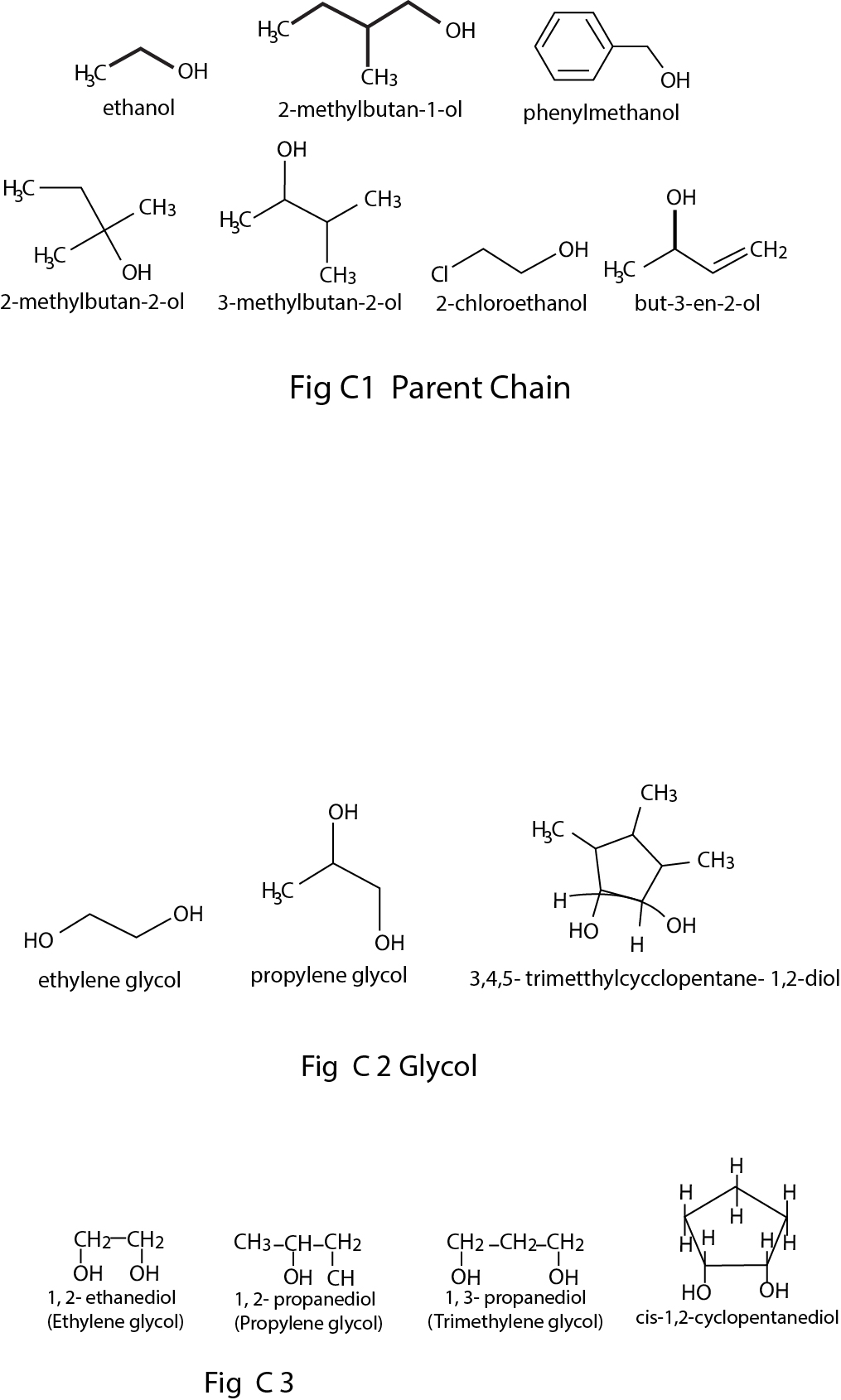
The organic compounds containing one or more than one hydroxyl group(s) attached to `sp^3` hybridised carbon atom(s) are called alcohols . Alcohols are bent shaped molecules.
In alcohols, the carbon atom linked with `O` atom of `- OH` group is `sp^3` hybridised. The central `O` atom is also `sp^3` hybridised and the `C - O - H` bond angle is `105^o`(fig.1).
Compounds in which the hydroxyl group is attached directly to a `sp^2` hybridised carbon atom are known as enols or phenols (in case of aromatic compounds)(fig.2).
In alcohols, the carbon atom linked with `O` atom of `- OH` group is `sp^3` hybridised. The central `O` atom is also `sp^3` hybridised and the `C - O - H` bond angle is `105^o`(fig.1).
Compounds in which the hydroxyl group is attached directly to a `sp^2` hybridised carbon atom are known as enols or phenols (in case of aromatic compounds)(fig.2).