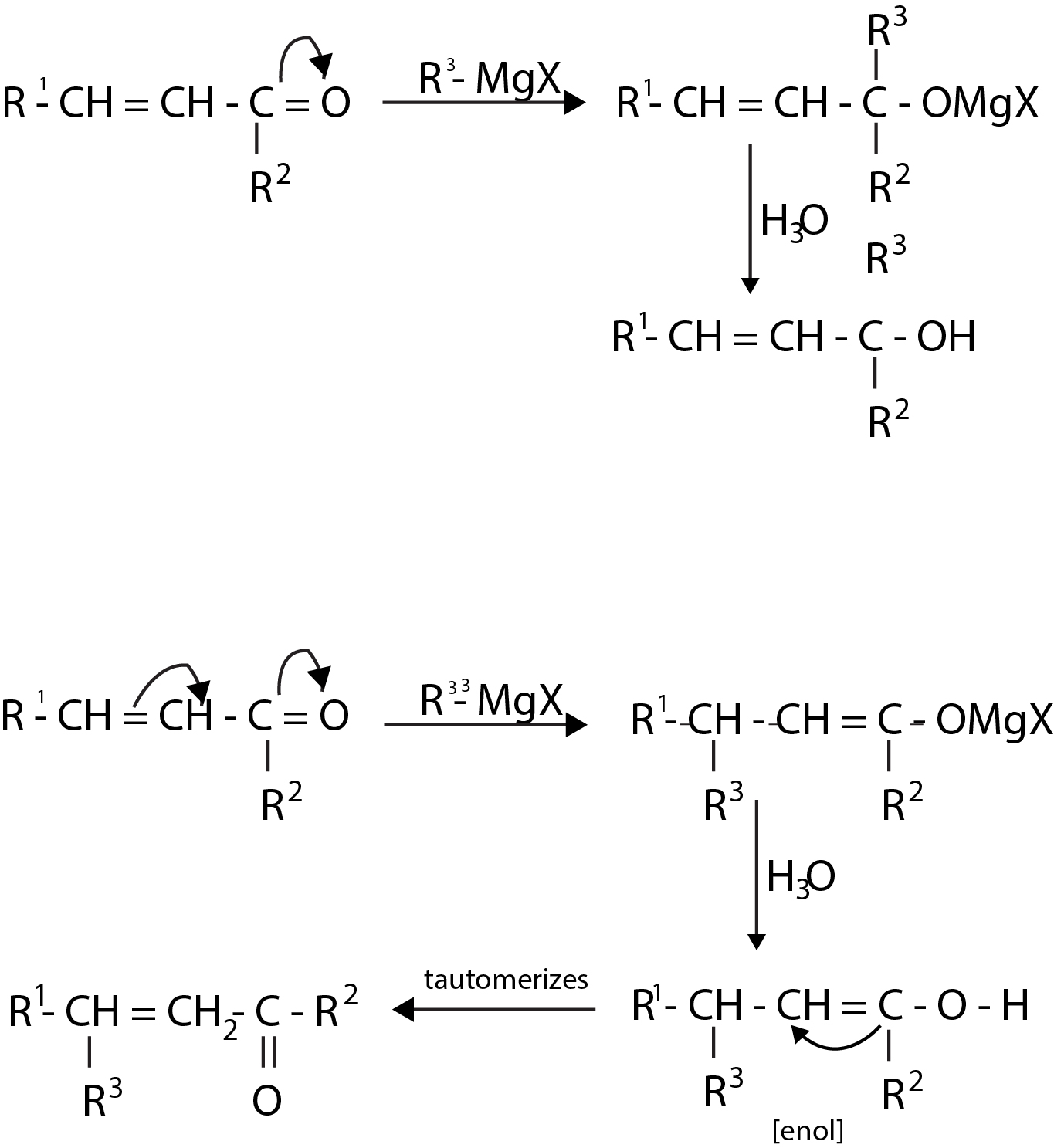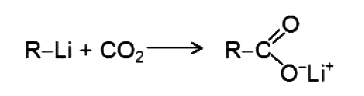When a solution of an alkyl halide in dry ethyl ether, `(C_2H_5)_2O`, is allowed to stand over turnings of metallic magnesium, a vigorous reaction takes place. The solution turns cloudy, begins to boil and the magnesium metal gradually disappears. The resulting solution is known as Grignard reagent. It is one of the most useful and versatile reagents known to the organic chemists.
`undersettext(Alkyl halide)(RX) + Mg oversettext(Dry ether ) (->) undersettext( Alkyl magnesium halide)(RMg X)`
The Grignard reagent has the general formula `RMgX`, and the general name alkyl magnesium halide. The carbon magnesium bond is covalent but highly polar, with carbon pulling away electrons from electropositive magnesium but the magnesium - halogen bond is essentially ionic, ` overset (-) (R) overset (+)(Mg) X`
Since magnesium becomes bonded to the same carbon that previously held halogen, the alkyl group remains intact during the preparation of the reagent. Thus, `n` - propyl chloride yields n - propyl magnesium chloride and isopropyl chloride yields isopropyl magnesium chloride.
`undersettext(n-Propyl chloride)(CH_3CH_2CH_2Cl) + Mg oversettext (Dry ether) (->) undersettext(
n - Propyl magnesium chloride)(CH_3CH_2CH_2MgCl)`
`undersettext(Isopropyl chloride)((CH_3)_2CHCl) + Mg oversettext(Dry ether) (->) undersettext( Isopropyl magnesium chloride)((CH_3)_2CHMgCl)`
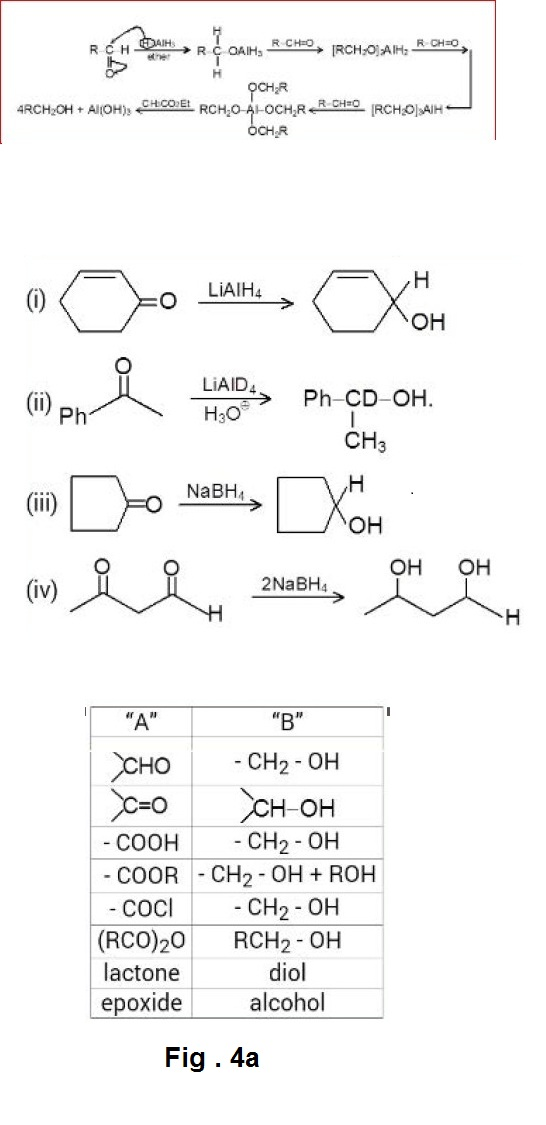
The Grignard reagent belongs to a class of compounds called organometallic compounds, in which carbon is bonded to a metal like lithium, potassium, sodium, zinc, mercury, lead, thallium or to almost any metal known. Each kind of organometallic compound has its own set of properties and its particular uses depend on these. But whatever the metal, it is less electronegative than carbon and the carbon - metal bond is always highly polar. Although the organic group is not a full - fledged carbanion but has considerable carbanionic characters. Thus, organometallic compounds can serve as a source of carbon bearing negative charge.
The Grignard reagent has the formula `RMgX` and is prepared by the reaction of metallic magnesium with the appropriate organic halide.
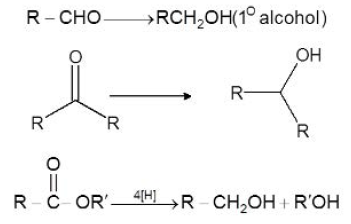
This halide can be alkyl `(1^o, 2^o, 3^o)`, allylic, aryl alkyl (e.g. benzyl), or aryl (phenyl) or substituted phenyl. The halogen may be -`Cl`, -`Br` or -`I`, (Aryl magnesium chlorides must be made in the cyclic ether tetrahydrofuran instead of ethyl ether). Aldehydes and ketones resemble each other closely in most of their reactions. The carbonyl group is also unsaturated and like the carbon - carbon bond, it also undergoes addition. One of the typical reaction is cis addition of the Grignard reagent.
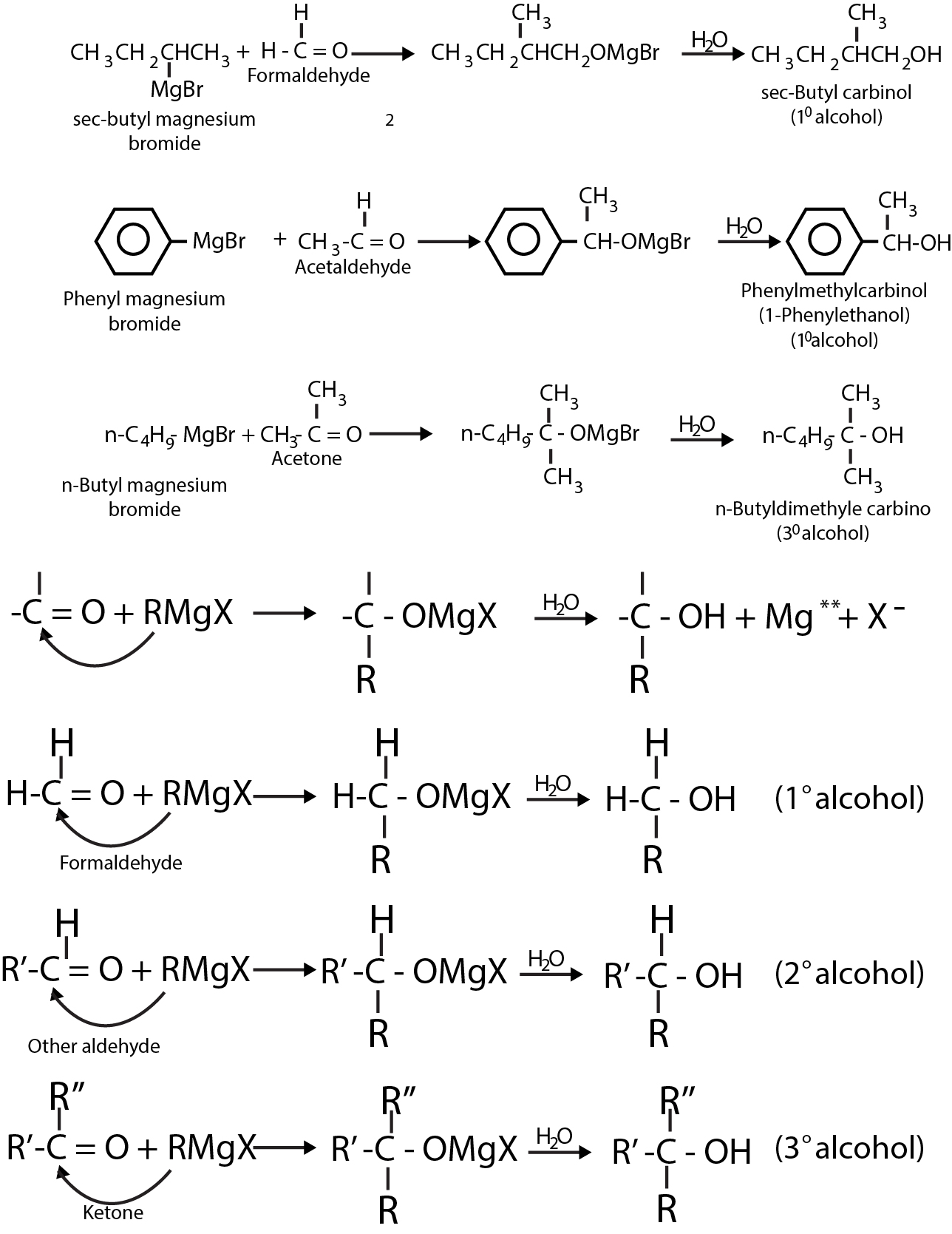
The electrons of the carbonyl double bond hold together atoms of different electronegativity, thus, the electrons are not equally shared, the mobile `p` - cloud is pulled strongly towards the more electronegative atom, oxygen. The addition of an unsymmetrical reagent happens such that the nucleophilic (basic) portion attaches itself to carbon and the electrophilic (acidic) portion attaches itself to oxygen.
The carbon - magnesium bond of the Grignard reagent is a highly polar bond, carbon being negative relative to electropositive magnesium. When Grignard reagent is added to carbonyl compounds, the organic group attaches to carbon and magnesium to oxygen.
The product is a magnesium salt of the weakly acidic alcohol and is easily converted into the alcohol by the addition of the stronger acid, water. The `Mg(OH)X` thus formed is a gelatinous material, which forms coating over carbonyl compound, thus dilute mineral acid
`(HCI, H_2SO_4)` is commonly used instead of water, so that water - soluble magnesium salts are formed.
When a solution of an alkyl halide in dry ethyl ether, `(C_2H_5)_2O`, is allowed to stand over turnings of metallic magnesium, a vigorous reaction takes place. The solution turns cloudy, begins to boil and the magnesium metal gradually disappears. The resulting solution is known as Grignard reagent. It is one of the most useful and versatile reagents known to the organic chemists.
`undersettext(Alkyl halide)(RX) + Mg oversettext(Dry ether ) (->) undersettext( Alkyl magnesium halide)(RMg X)`
The Grignard reagent has the general formula `RMgX`, and the general name alkyl magnesium halide. The carbon magnesium bond is covalent but highly polar, with carbon pulling away electrons from electropositive magnesium but the magnesium - halogen bond is essentially ionic, ` overset (-) (R) overset (+)(Mg) X`
Since magnesium becomes bonded to the same carbon that previously held halogen, the alkyl group remains intact during the preparation of the reagent. Thus, `n` - propyl chloride yields n - propyl magnesium chloride and isopropyl chloride yields isopropyl magnesium chloride.
`undersettext(n-Propyl chloride)(CH_3CH_2CH_2Cl) + Mg oversettext (Dry ether) (->) undersettext(
n - Propyl magnesium chloride)(CH_3CH_2CH_2MgCl)`
`undersettext(Isopropyl chloride)((CH_3)_2CHCl) + Mg oversettext(Dry ether) (->) undersettext( Isopropyl magnesium chloride)((CH_3)_2CHMgCl)`
The Grignard reagent belongs to a class of compounds called organometallic compounds, in which carbon is bonded to a metal like lithium, potassium, sodium, zinc, mercury, lead, thallium or to almost any metal known. Each kind of organometallic compound has its own set of properties and its particular uses depend on these. But whatever the metal, it is less electronegative than carbon and the carbon - metal bond is always highly polar. Although the organic group is not a full - fledged carbanion but has considerable carbanionic characters. Thus, organometallic compounds can serve as a source of carbon bearing negative charge.
The Grignard reagent has the formula `RMgX` and is prepared by the reaction of metallic magnesium with the appropriate organic halide.
This halide can be alkyl `(1^o, 2^o, 3^o)`, allylic, aryl alkyl (e.g. benzyl), or aryl (phenyl) or substituted phenyl. The halogen may be -`Cl`, -`Br` or -`I`, (Aryl magnesium chlorides must be made in the cyclic ether tetrahydrofuran instead of ethyl ether). Aldehydes and ketones resemble each other closely in most of their reactions. The carbonyl group is also unsaturated and like the carbon - carbon bond, it also undergoes addition. One of the typical reaction is cis addition of the Grignard reagent.
The electrons of the carbonyl double bond hold together atoms of different electronegativity, thus, the electrons are not equally shared, the mobile `p` - cloud is pulled strongly towards the more electronegative atom, oxygen. The addition of an unsymmetrical reagent happens such that the nucleophilic (basic) portion attaches itself to carbon and the electrophilic (acidic) portion attaches itself to oxygen.
The carbon - magnesium bond of the Grignard reagent is a highly polar bond, carbon being negative relative to electropositive magnesium. When Grignard reagent is added to carbonyl compounds, the organic group attaches to carbon and magnesium to oxygen.
The product is a magnesium salt of the weakly acidic alcohol and is easily converted into the alcohol by the addition of the stronger acid, water. The `Mg(OH)X` thus formed is a gelatinous material, which forms coating over carbonyl compound, thus dilute mineral acid
`(HCI, H_2SO_4)` is commonly used instead of water, so that water - soluble magnesium salts are formed.