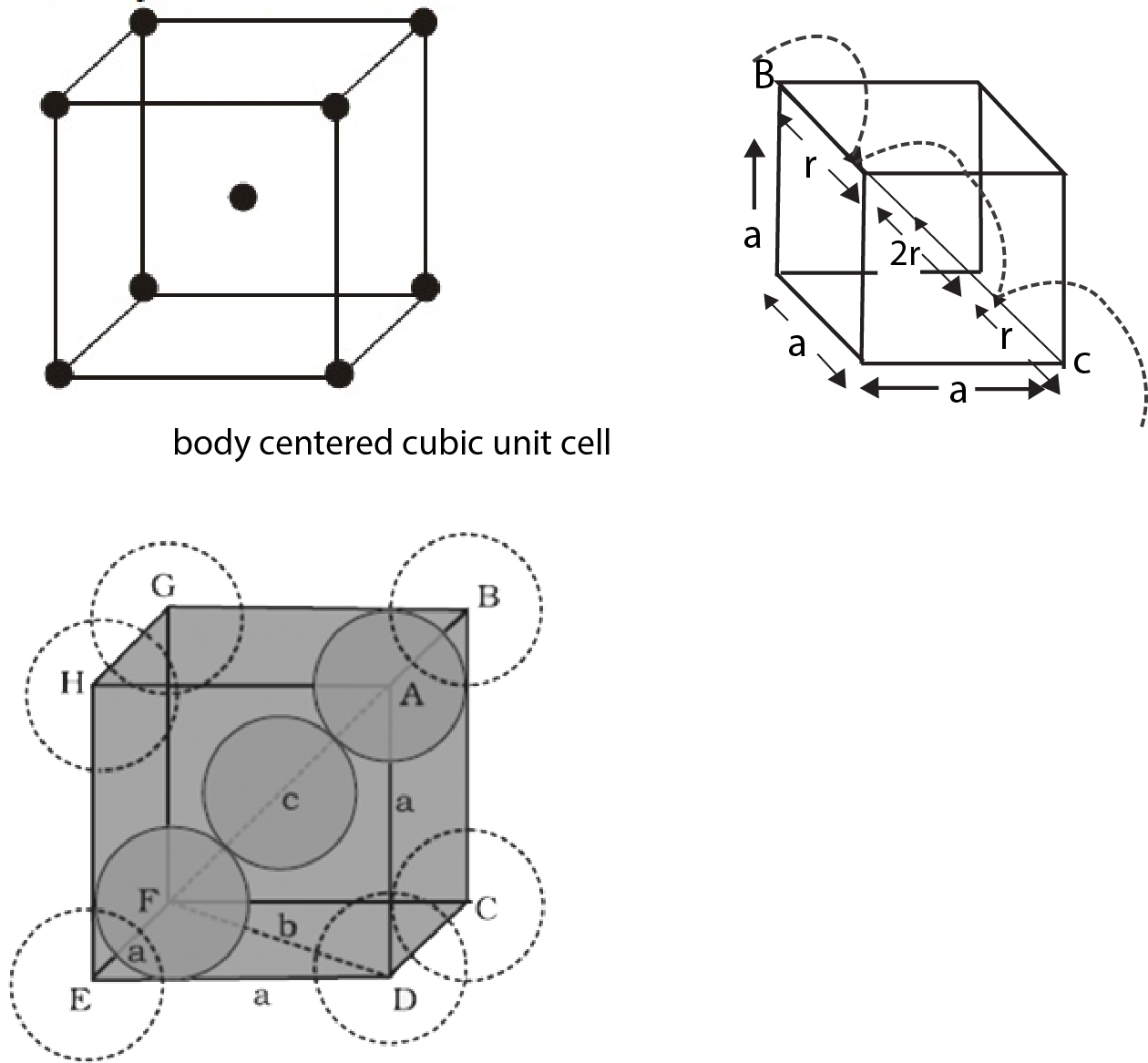
Primitive Cubic Unit Cell :
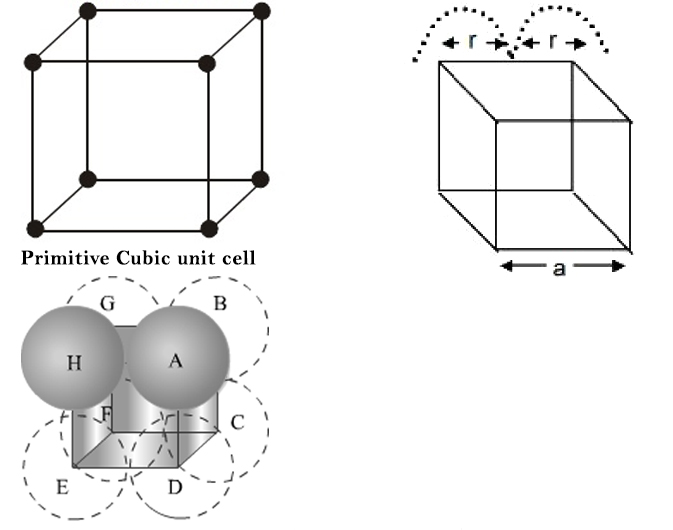
It is defined as ratio of the volume occupied by the spheres in a unit cell to the volume of the unit cell. Thus void fraction is given as `= (1 - text(Packing fraction))`. Since adjacent atoms touch each other. the edge length of the unit cell `'a'` is equal to `2r`, where `r` is the radius of the sphere.
Therefore, Packing fraction `(PF) = (4/3 pi r^3)/((2r)^3) approx 0.52` (This implies that `52%` of the volume of a unit cell is occupied by spheres).
Void Fraction `(VF) approx 0.48`
Therefore, Packing fraction `(PF) = (4/3 pi r^3)/((2r)^3) approx 0.52` (This implies that `52%` of the volume of a unit cell is occupied by spheres).
Void Fraction `(VF) approx 0.48`