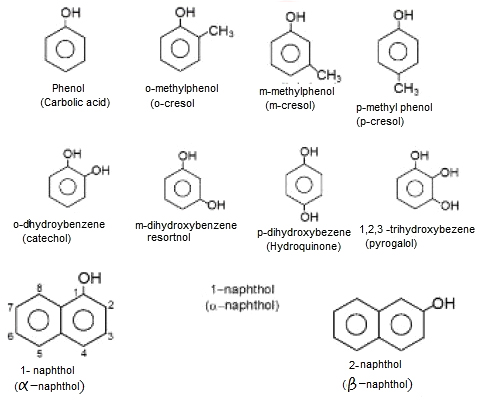
(i) Phenol is a colorless, toxic, corrosive, needle shaped solid.
(ii) Phenol soon liquifies due to high hygroscopic nature.
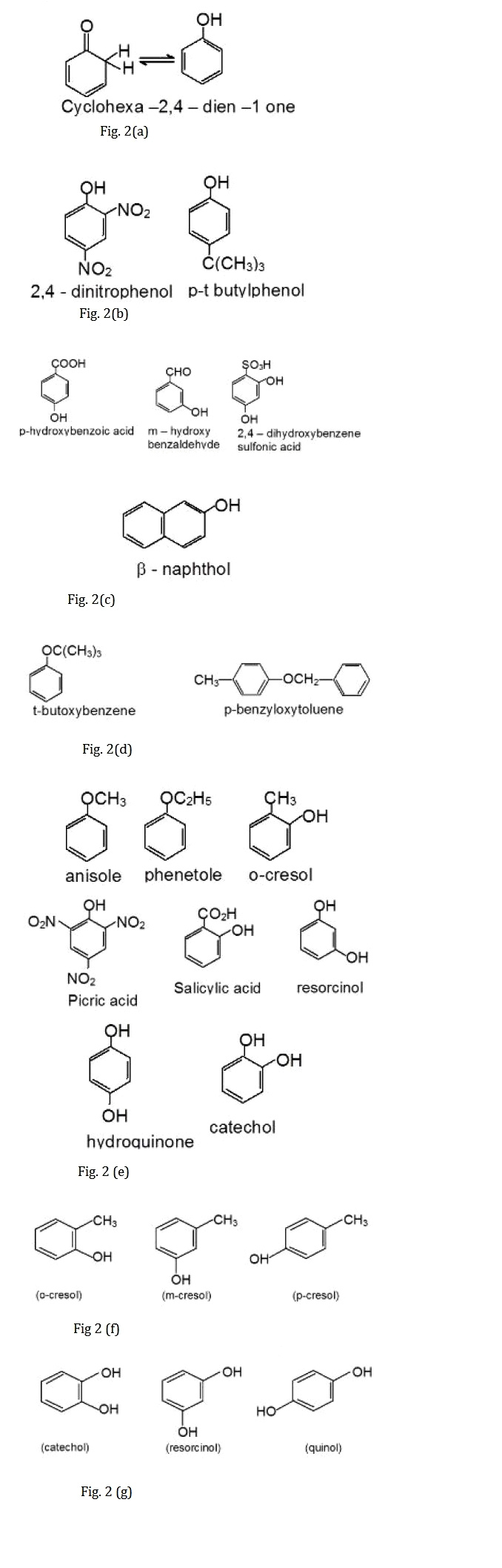
Unlike simple ketones, which are far more stable than their corresponding enols, the analogous equilibrium for phenol lies far on the side of the enol form which is shown in Fig.2(a). The reason for this difference is the resonance energy of the aromatic ring, which provides an important stabilization of the enol form.
Since the functional group occurs as suffix in phenol, many compounds containing hydroxyl group are named as derivatives of the parent compound phenol, as illustrated by the IUPAC names as shown in Fig.2(b).
Suffix groups such as sulfonic acid and carboxylic acid take priority, and when these groups are present the hydroxyl group is used as a modifying prefix as shown in Fig.2(c).
Phenyl ethers are named in the IUPAC system as alkoxyarenes, although the ether nomenclature is used for some compounds shown in Fig.2(d).
Phenols and their ethers are widespread in nature, and, as is usual for such compounds, trivial names abound shown in Fig. 2(e).
The methyl phenols are commonly called cresols in Fig.2(f).
The benzene diols also have common names as shown in Fig.2(g).
(i) Phenol is a colorless, toxic, corrosive, needle shaped solid.
(ii) Phenol soon liquifies due to high hygroscopic nature.
Unlike simple ketones, which are far more stable than their corresponding enols, the analogous equilibrium for phenol lies far on the side of the enol form which is shown in Fig.2(a). The reason for this difference is the resonance energy of the aromatic ring, which provides an important stabilization of the enol form.
Since the functional group occurs as suffix in phenol, many compounds containing hydroxyl group are named as derivatives of the parent compound phenol, as illustrated by the IUPAC names as shown in Fig.2(b).
Suffix groups such as sulfonic acid and carboxylic acid take priority, and when these groups are present the hydroxyl group is used as a modifying prefix as shown in Fig.2(c).
Phenyl ethers are named in the IUPAC system as alkoxyarenes, although the ether nomenclature is used for some compounds shown in Fig.2(d).
Phenols and their ethers are widespread in nature, and, as is usual for such compounds, trivial names abound shown in Fig. 2(e).
The methyl phenols are commonly called cresols in Fig.2(f).
The benzene diols also have common names as shown in Fig.2(g).