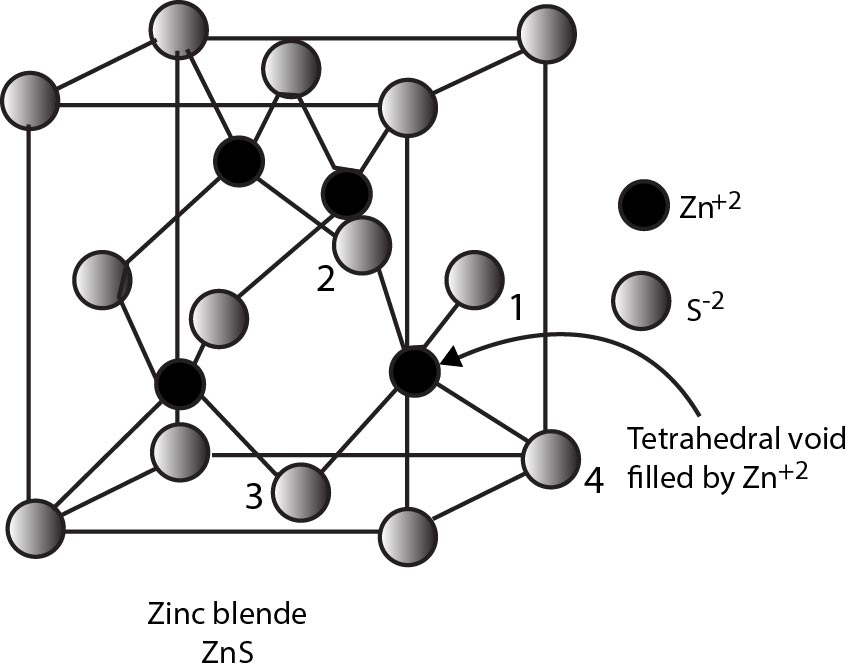
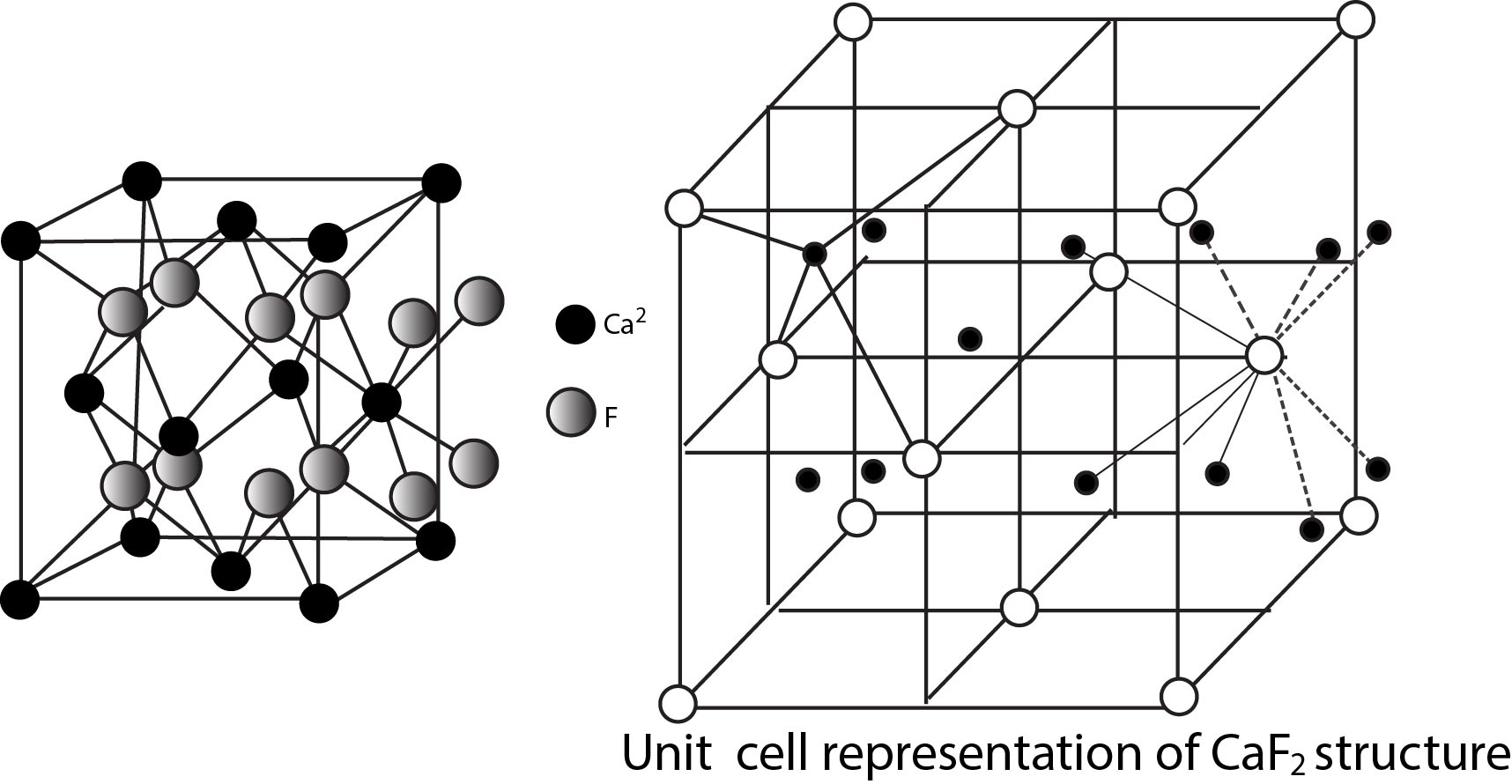
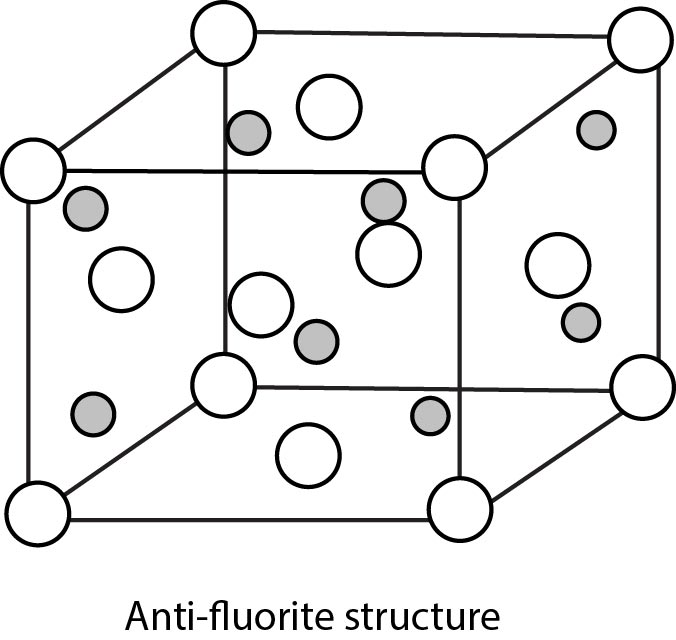
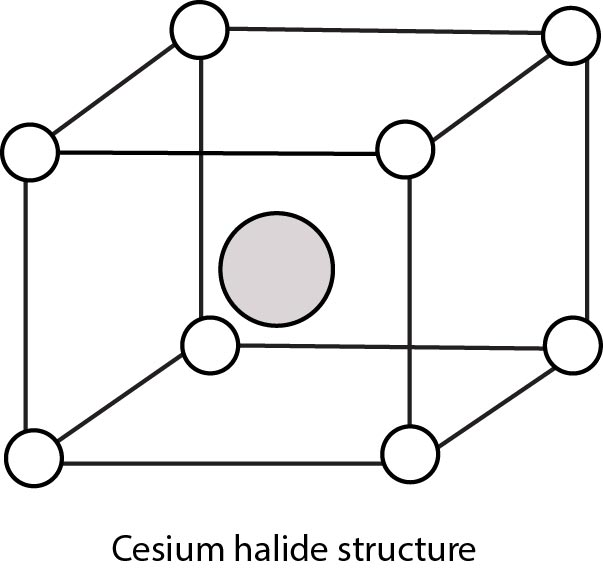
Rock Salt Structure :
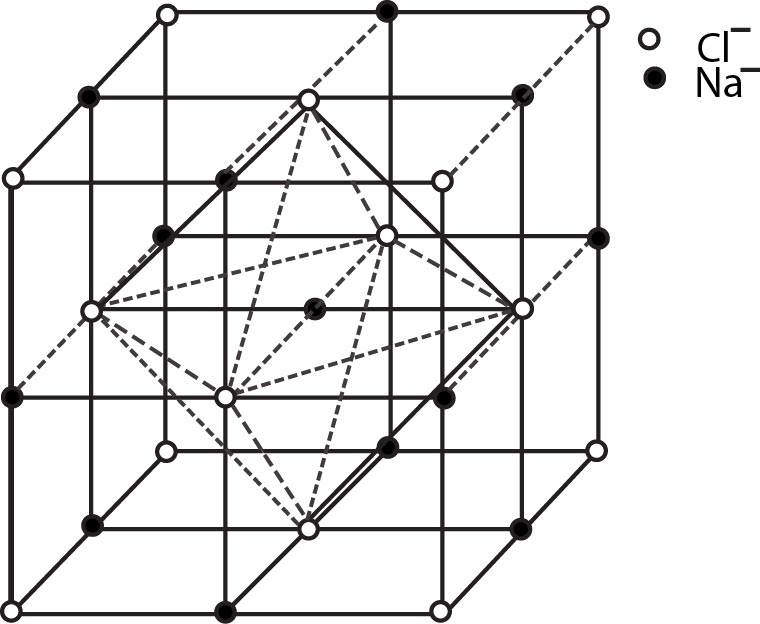
`NaCl` exhibits this type of structure. In the rock salt structure, `Cl^(-)` ions exists in FCC pattern and `Na^+` ions occupy all octahedral voids. There are `4` effective `Na^+` ions and `4` effective `Cl^(-)` ions in a unit cell of `NaCl`. So, the general formula is `Na_4Cl_4` or `NaCl` as per the effective ions in a unit cell. The co - ordination number of `Na^+` ion is `6` and co- ordination number of `Cl^(-)` ion is also `6`. So, the general formula (using co - ordination number of ions) is `Na_6Cl_6` or `NaCl `....The other substances having this kind of a structure are halides of all alkali metals except cesium halides and oxides of all alkaline earth metals except beryllium oxide.