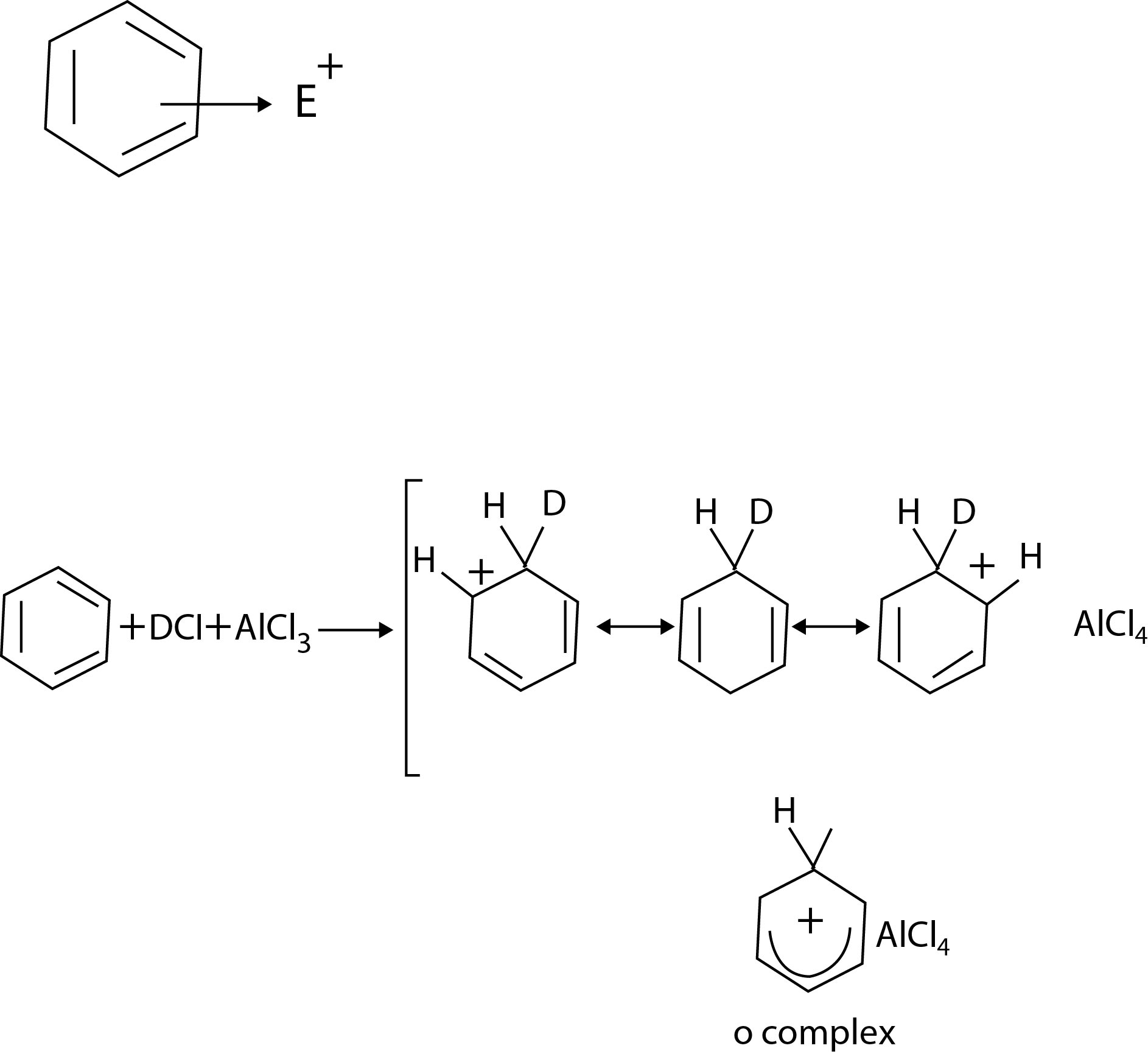
Electrophilic Aromatic Substitution Reactions :

Aromatic hydrocarbons are known generally as arenes. An aryl group is one derived from an arene by removal of a hydrogen atom and its symbol is Ar -. Thus, arenes are designated `ArH` just as alkanes are designated `RH`. The most characteristic reactions of benzenoid arenes are the substitution reactions that occur when they react with electrophilic reagents. These reactions are of the general type shown below.
`ArH+E^(+)-> Ar-E+H^(+)`
The electrophiles are ei ther a positive ion (`E^(+)`) or some other electron -deficient species with a large partial positive charge. For xample, benzene can be brominated when it reacts with bromine in the presence of `FeBr_3`. Bromine and `FeBr_3` reacts to produce positive bromonium ions, `Br^(+)`. These positive bromonium ions act as electrophiles and attack the benzene ring replacing one of the hydrogen atoms in a reaction that is called an electrophilic aromatic substitution (`EAS`). Electrophilic aromatic substitutions allow the direct introduction of a wide variety of groups into an aromatic ring and because of this they provide synthetic routes to many important compounds. The five electrophilic aromatic substitutions that we shall study in this package are outlined in fig. All of these reactions involve the attack on the benzene ring by an electron-deficient species - (by an electrophile). Later we shall learn what the electrophile is in each instance
`ArH+E^(+)-> Ar-E+H^(+)`
The electrophiles are ei ther a positive ion (`E^(+)`) or some other electron -deficient species with a large partial positive charge. For xample, benzene can be brominated when it reacts with bromine in the presence of `FeBr_3`. Bromine and `FeBr_3` reacts to produce positive bromonium ions, `Br^(+)`. These positive bromonium ions act as electrophiles and attack the benzene ring replacing one of the hydrogen atoms in a reaction that is called an electrophilic aromatic substitution (`EAS`). Electrophilic aromatic substitutions allow the direct introduction of a wide variety of groups into an aromatic ring and because of this they provide synthetic routes to many important compounds. The five electrophilic aromatic substitutions that we shall study in this package are outlined in fig. All of these reactions involve the attack on the benzene ring by an electron-deficient species - (by an electrophile). Later we shall learn what the electrophile is in each instance