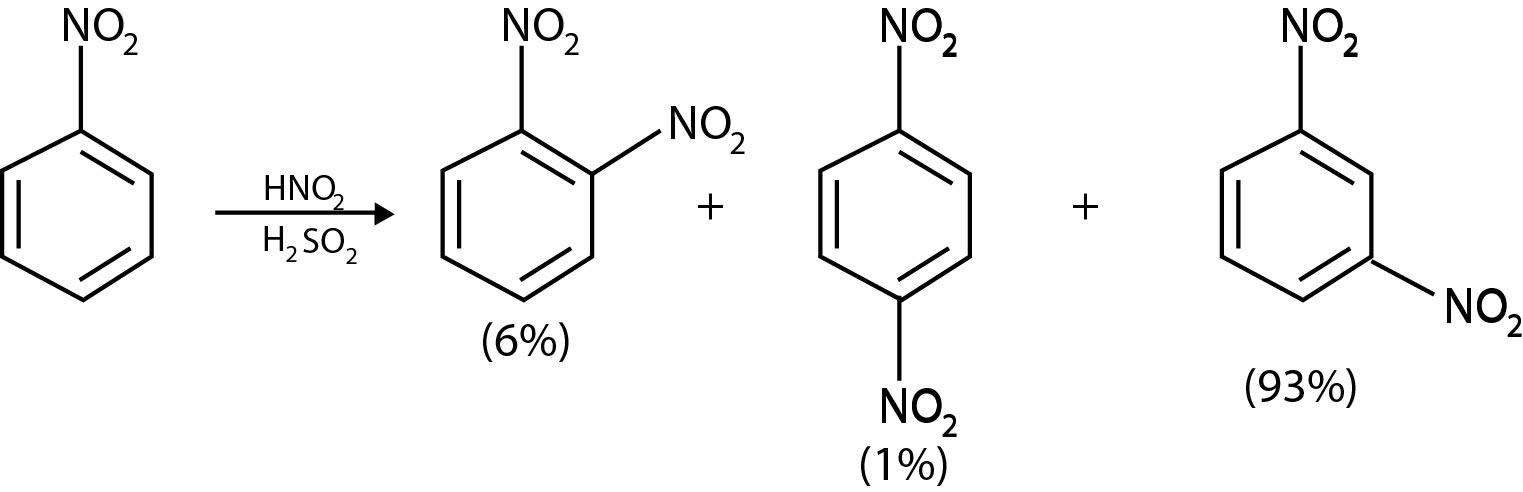
We can account for the electron-withdrawing and electron - releasing properties of a group on the basis of two factors: inductive effect and resonance effects. We shall also see that these two factors determine orientation in aromatic substitution reactions. The inductive effect of a substituent `S` arises from the electrostatic interaction of the polarized `S` to ring bond with the developing positive charge in the ring as it is attacked by an electrophile. If, for example, `S` is a more electronegative atom (or group) than carbon, then the ring will be at the end of the dipole:
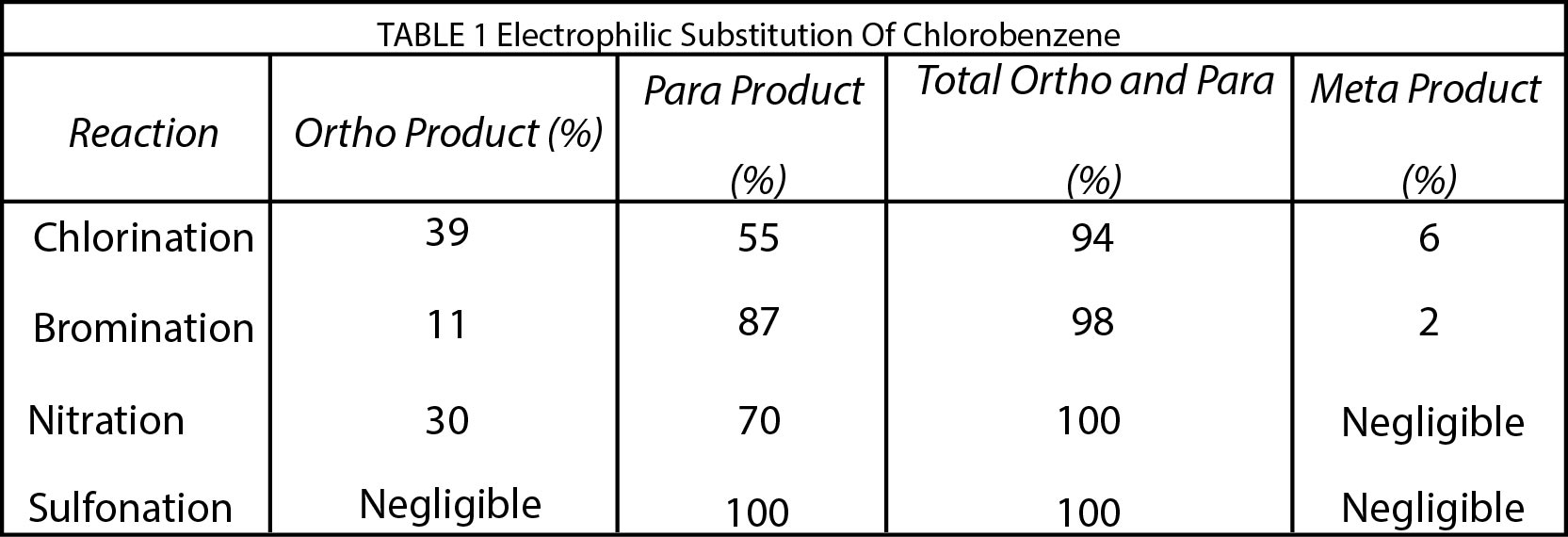
Attack by an electrophile will be retarded because this will lead to an additional full positive charge on the ring. The halogens are all more electronegative than carbon and exert an electron - withdrawing inductive effect. Other groups have an electron - withdrawing inductive effect because the atom directly attached to the ring bears a full or partial positive charge. Examples are the following:
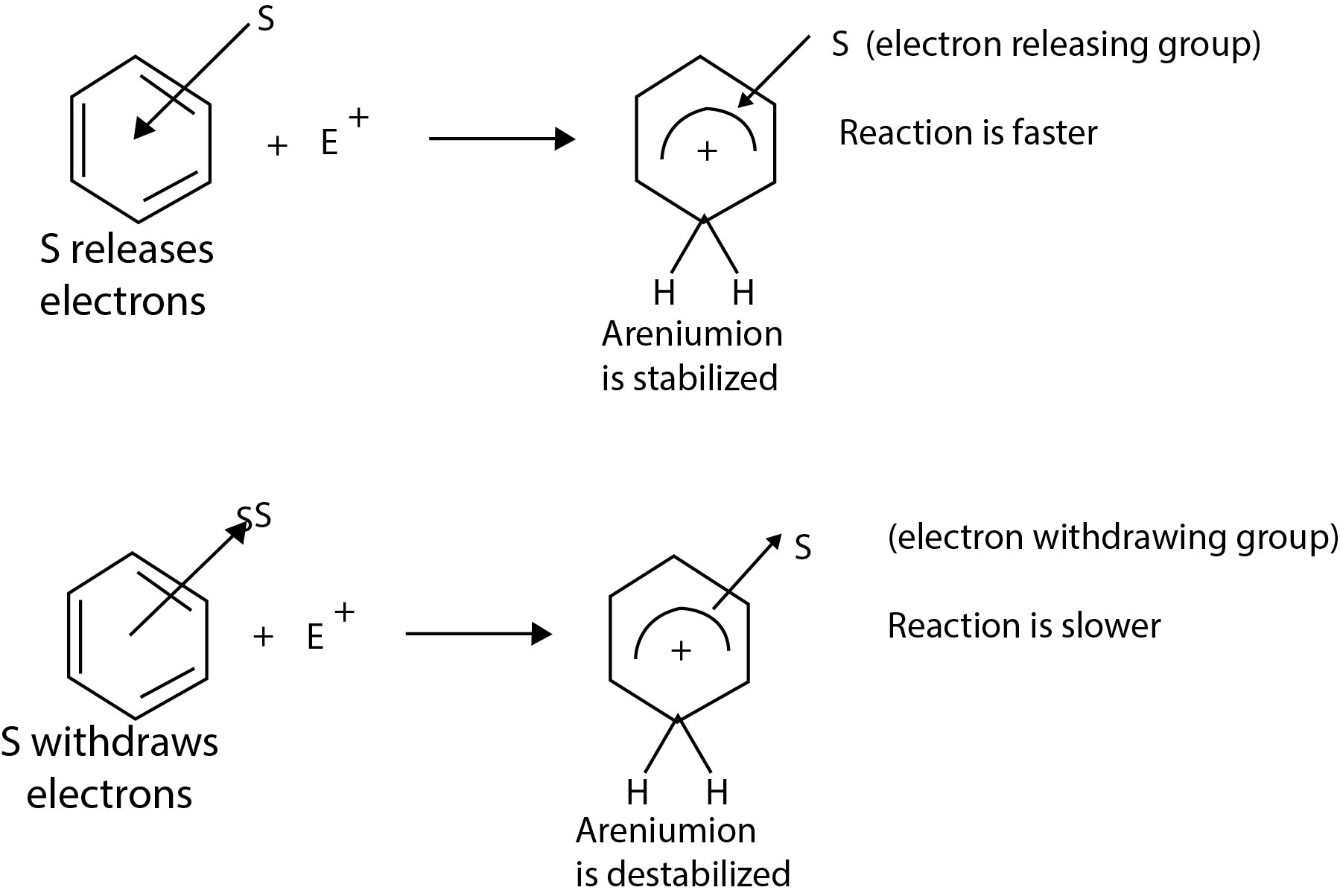
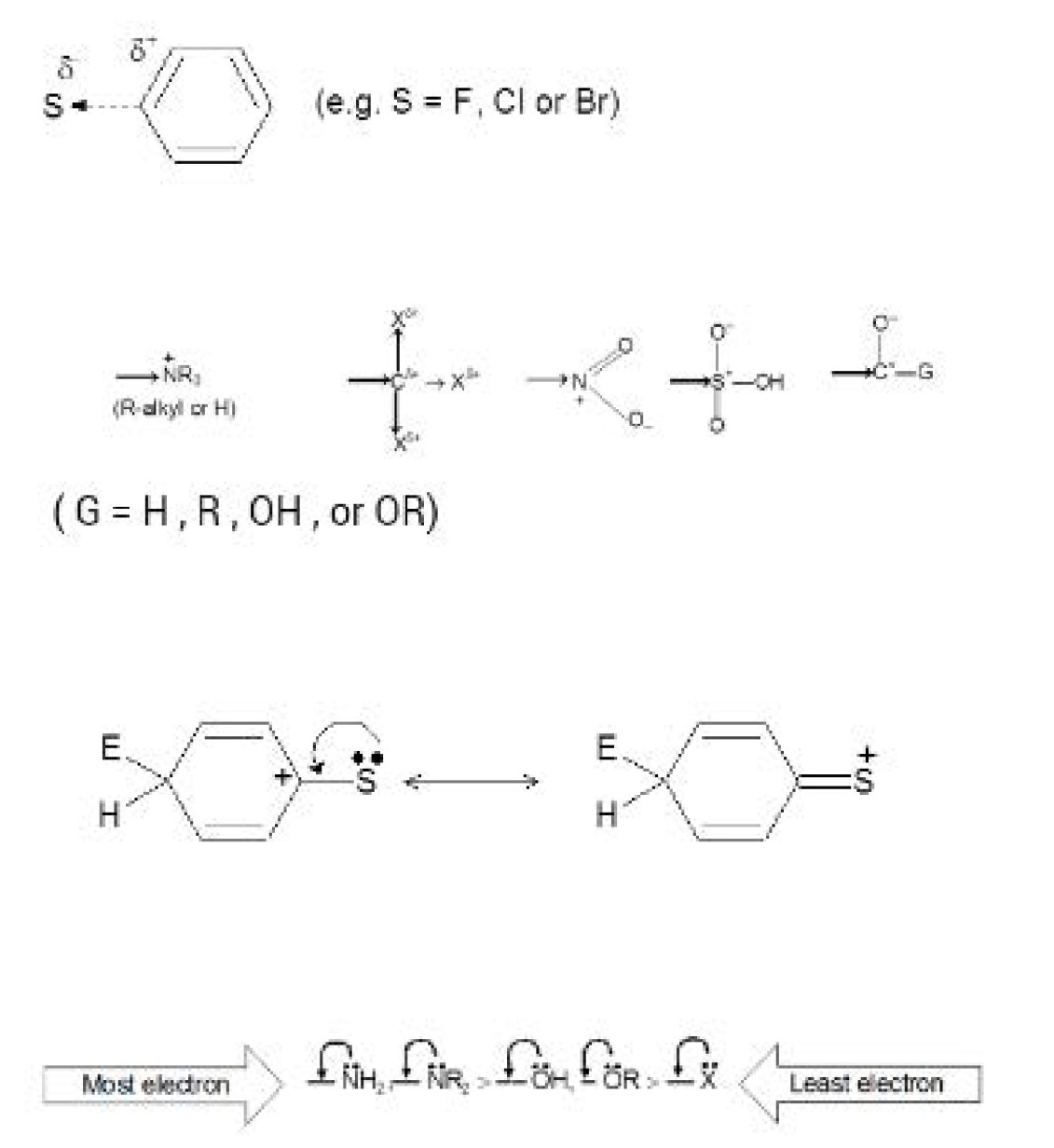
Then resonance effect of a substituent `S` refers to the possibility that the presence of `S` may increase or decrease the resonance stabilization of the intermediate areniumion. The `S` substituent may, for example cause one of the three contributors to the resonance hybrid for the areniumion to be stabilized or destabilized than the case when `S` is hydrogen. Moreover, when `S` is an atom bearing one or more nonbonding electron pairs, it may lend extra stability to the areniumion by providing a fourth resonance contributor in which the positive charge resides on `S`.This electron-donating resonance effect applies with decreasing strength in the following order:
This is also the order of the activating ability of these groups. Amino groups are highly activating, hydroxyl and alkoxyl groups are somewhat less activating, and halogen substituents are weakly deactivating. When `X= F`, this order can be related to the lectronegativity of the atoms with the nonbonding pair. The more electronegative the atom is the less able it is to accept the positive charge (f luorine is the most electronegative, nitrogen the least). When `X = Cl, Br`, or `I`, the relatively poor electron-donating ability of the halogens by resonance is understandable on a different basis. These atoms (`CI, Br`, and `I`) are all larger than carbon and, therefore, the orbitals that contain the nonbonding pairs are further from the nucleus and do not overlap well with the `2p` orbital of carbon. (This is a general phenomenon: resonance effects are not transmitted well between atoms of different rows in the periodic table.)
We can account for the electron-withdrawing and electron - releasing properties of a group on the basis of two factors: inductive effect and resonance effects. We shall also see that these two factors determine orientation in aromatic substitution reactions. The inductive effect of a substituent `S` arises from the electrostatic interaction of the polarized `S` to ring bond with the developing positive charge in the ring as it is attacked by an electrophile. If, for example, `S` is a more electronegative atom (or group) than carbon, then the ring will be at the end of the dipole:
Attack by an electrophile will be retarded because this will lead to an additional full positive charge on the ring. The halogens are all more electronegative than carbon and exert an electron - withdrawing inductive effect. Other groups have an electron - withdrawing inductive effect because the atom directly attached to the ring bears a full or partial positive charge. Examples are the following:
Then resonance effect of a substituent `S` refers to the possibility that the presence of `S` may increase or decrease the resonance stabilization of the intermediate areniumion. The `S` substituent may, for example cause one of the three contributors to the resonance hybrid for the areniumion to be stabilized or destabilized than the case when `S` is hydrogen. Moreover, when `S` is an atom bearing one or more nonbonding electron pairs, it may lend extra stability to the areniumion by providing a fourth resonance contributor in which the positive charge resides on `S`.This electron-donating resonance effect applies with decreasing strength in the following order:
This is also the order of the activating ability of these groups. Amino groups are highly activating, hydroxyl and alkoxyl groups are somewhat less activating, and halogen substituents are weakly deactivating. When `X= F`, this order can be related to the lectronegativity of the atoms with the nonbonding pair. The more electronegative the atom is the less able it is to accept the positive charge (f luorine is the most electronegative, nitrogen the least). When `X = Cl, Br`, or `I`, the relatively poor electron-donating ability of the halogens by resonance is understandable on a different basis. These atoms (`CI, Br`, and `I`) are all larger than carbon and, therefore, the orbitals that contain the nonbonding pairs are further from the nucleus and do not overlap well with the `2p` orbital of carbon. (This is a general phenomenon: resonance effects are not transmitted well between atoms of different rows in the periodic table.)