Meta-Directing Groups :
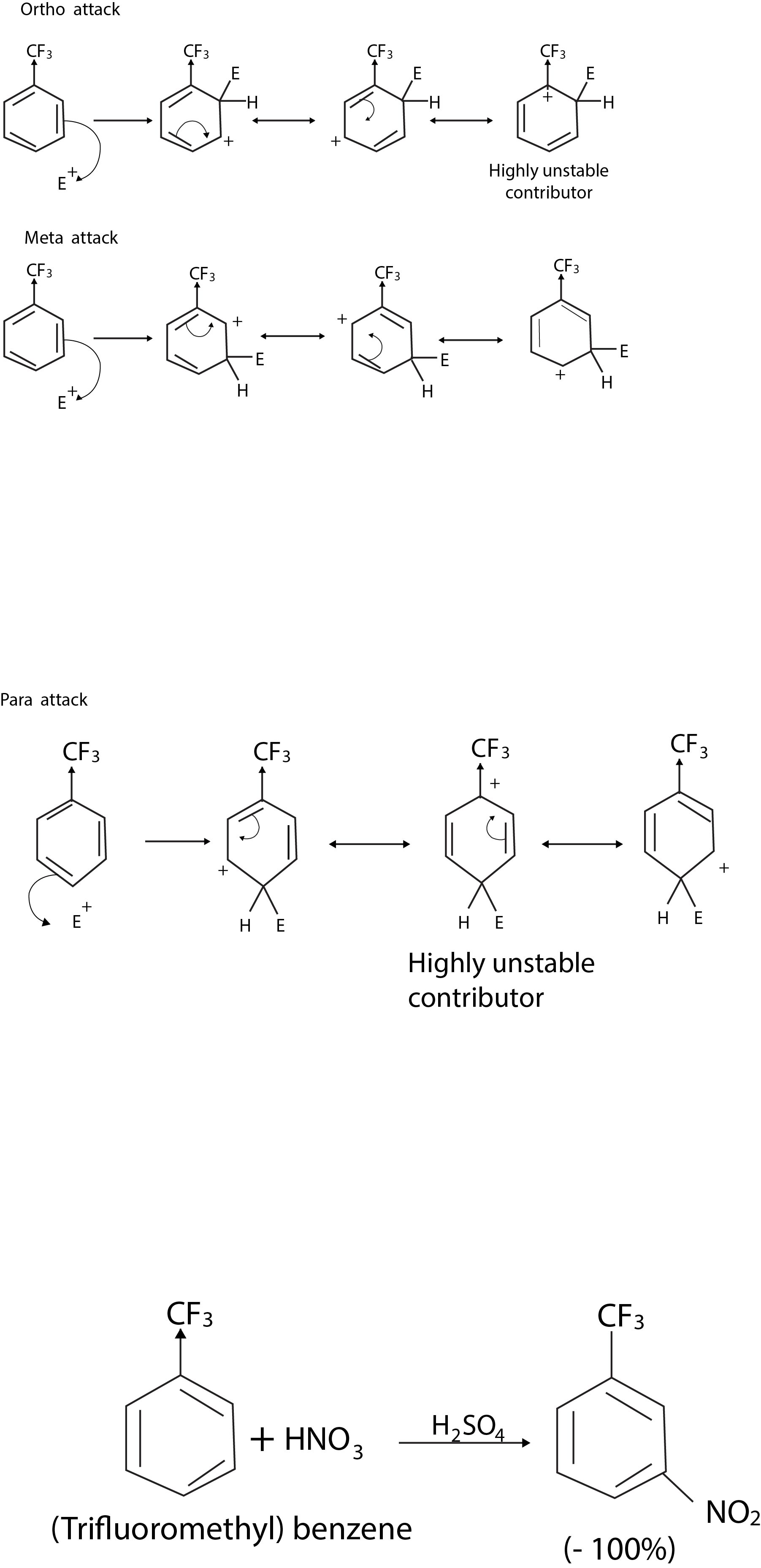
All meta-directing groups have either a partial positive charge or a full positive charge on the atom directly attached to the ring. As a typical example let us consider the trifluoromethyl group. The trifluoromethyl group, because of the three highly electronegative fluorine atoms, is strongly electron withdrawing. It is a strong deactivating group and a powerful meta director in electrophilic aromatic substitution reactions. We can account for both of these characteristics of the trifluoromethyl group in the following way. The trifluoromethyl group affects reactivity by causing the transition state leading to the areniumion to be highly unstable. It does this by withdrawing electrons from the developing carbocation thus increasing the positive charge in the ring. We can understand how the trifluoromethyl group affects orientation in electrophilic aromatic substitution if we examine the resonance structures for the arenium ion that would be formed when an electrophile attacks the ortho, meta, and para positions of (trifluoromethyl) benzene.
We see in the resonance structures for the arenium ion arising from ortho and para attack that one contributing structure is highly unstable relative to all the others because the positive charge is located on the ring carbon that bears the electron-withdrawing group. We see no such highly unstable resonance structure in the arenium ion arising from meta attack. This means that the arenium ion formed by meta attack should be the most stable of the three. By the usual reasoning we would also expect the transition state leading to the meta - substituted arenium ion to be the most stable and, therefore, that meta attack would be favoured. This is exactly what we find experimentally. The trifluoromethyl group is a powerful meta director.
We bear in mind, however, that meta substitution is favoured only in the sense that it is the least unfavorable of three unfavourable pathways. The free energy of activation for substitution at the meta position of (trifluoromethyl) benzene is less than that for attack at an ortho or para position, but it is still far greater than that for an attack on benzene. Substitution occurs at the meta position of trifluoromethyl) benzene faster than substitution takes place at the ortho and para positions, but it occurs much more slowly than it does with benzene. The nitro group, the carboxyl group, and other metadirecting groups are all powerful electron-withdrawing groups and all act in similar way.
We see in the resonance structures for the arenium ion arising from ortho and para attack that one contributing structure is highly unstable relative to all the others because the positive charge is located on the ring carbon that bears the electron-withdrawing group. We see no such highly unstable resonance structure in the arenium ion arising from meta attack. This means that the arenium ion formed by meta attack should be the most stable of the three. By the usual reasoning we would also expect the transition state leading to the meta - substituted arenium ion to be the most stable and, therefore, that meta attack would be favoured. This is exactly what we find experimentally. The trifluoromethyl group is a powerful meta director.
We bear in mind, however, that meta substitution is favoured only in the sense that it is the least unfavorable of three unfavourable pathways. The free energy of activation for substitution at the meta position of (trifluoromethyl) benzene is less than that for attack at an ortho or para position, but it is still far greater than that for an attack on benzene. Substitution occurs at the meta position of trifluoromethyl) benzene faster than substitution takes place at the ortho and para positions, but it occurs much more slowly than it does with benzene. The nitro group, the carboxyl group, and other metadirecting groups are all powerful electron-withdrawing groups and all act in similar way.