Introduction of a `3^(rd)` group into the benzene ring :
The position taken up by a third electrophile entering the ring depends on the nature of the two groups already present. It is often possible in such cases to predict the correct isomer: but remember following generalizations in order to do so.
1. When the two groups direct differently, i.e. they belong to the classes I and II . then class I group takes precedence.
In the following examples, the number of arrowheads indicates (qualitatively) the amount of substitution and the encircled number below indicates the possible number of isomers. For example fig-1.
2. When both groups belong to class I then introduction of third group is very easy and the group enters in accordance with higher activating group. For this purpose. we can arrange the groups in the following order: `O^(-), NH_2, OH, NR_2, OR > OCOR, NHCOR
> R, Ar > ` halogen. For example, fig-2.
3. When both groups belong to class II , then it is difficult to introduce a third group and the deactivating power of groups is in the order:
`Me_3N^(+) > NO_2 > CN > SO_3H > CHO > COMe > CO_2H`
The most deactivating group controls the orientation. For example, fig-3.
4. All other things being equal, a third group is least likely to enter between two groups in the meta position. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking electrophile. For example, fig-4
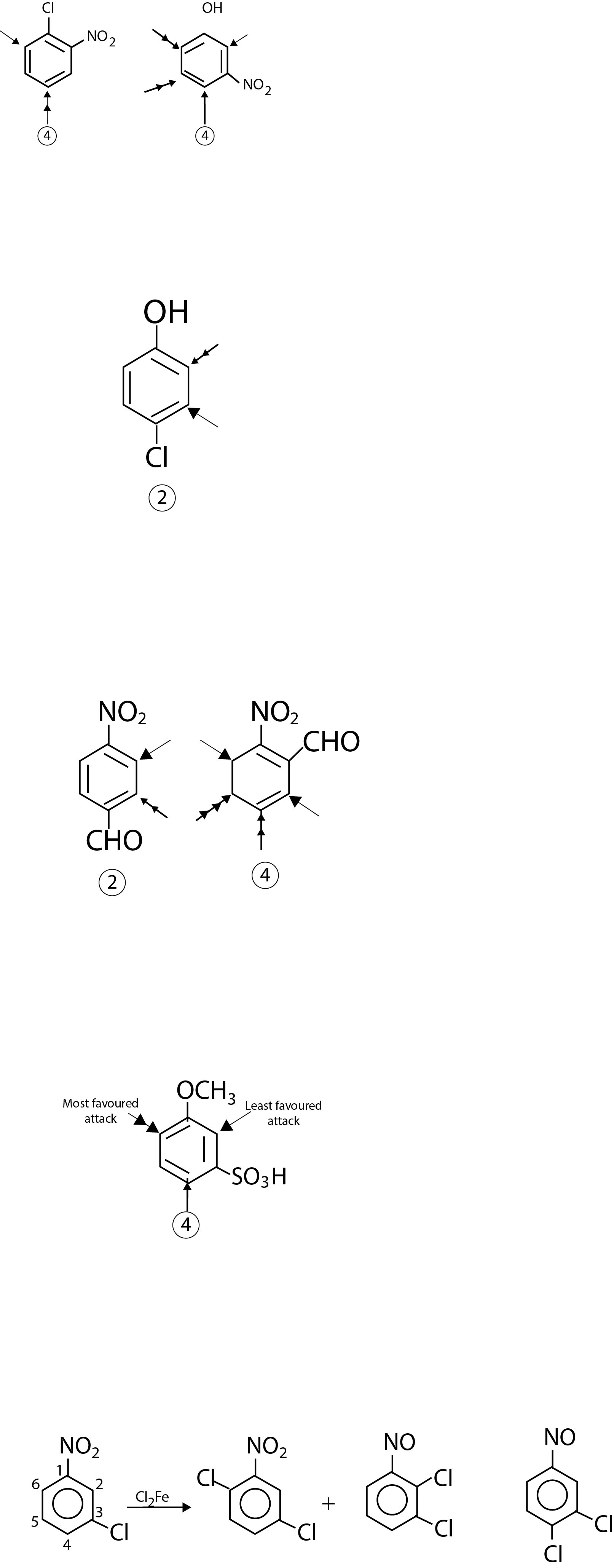
5. When a meta ∑ directing group is meta to an ortho/para - directing group, the incoming group primarily goes ortho to the meta-directing group rather than para. For example, chlorination of m - chloronitrobenzene ( I ) mostly gives ( II ) and small amounts of ( Ill ) but ( IV) is formed in very small amount. fig-5
It is interesting that chlorination of (I) illustrates three rules. Of the four positions available to electrophile, the position-S violates rule 1, position-2 violates rule 4 and position-4 disregards rule 5. Thus, principal attack of Cl A is taking place at position-6 giving ( II ) as the major product.
1. When the two groups direct differently, i.e. they belong to the classes I and II . then class I group takes precedence.
In the following examples, the number of arrowheads indicates (qualitatively) the amount of substitution and the encircled number below indicates the possible number of isomers. For example fig-1.
2. When both groups belong to class I then introduction of third group is very easy and the group enters in accordance with higher activating group. For this purpose. we can arrange the groups in the following order: `O^(-), NH_2, OH, NR_2, OR > OCOR, NHCOR
> R, Ar > ` halogen. For example, fig-2.
3. When both groups belong to class II , then it is difficult to introduce a third group and the deactivating power of groups is in the order:
`Me_3N^(+) > NO_2 > CN > SO_3H > CHO > COMe > CO_2H`
The most deactivating group controls the orientation. For example, fig-3.
4. All other things being equal, a third group is least likely to enter between two groups in the meta position. This is the result of steric hindrance and increases in importance with the size of the groups on the ring and with the size of the attacking electrophile. For example, fig-4
5. When a meta ∑ directing group is meta to an ortho/para - directing group, the incoming group primarily goes ortho to the meta-directing group rather than para. For example, chlorination of m - chloronitrobenzene ( I ) mostly gives ( II ) and small amounts of ( Ill ) but ( IV) is formed in very small amount. fig-5
It is interesting that chlorination of (I) illustrates three rules. Of the four positions available to electrophile, the position-S violates rule 1, position-2 violates rule 4 and position-4 disregards rule 5. Thus, principal attack of Cl A is taking place at position-6 giving ( II ) as the major product.