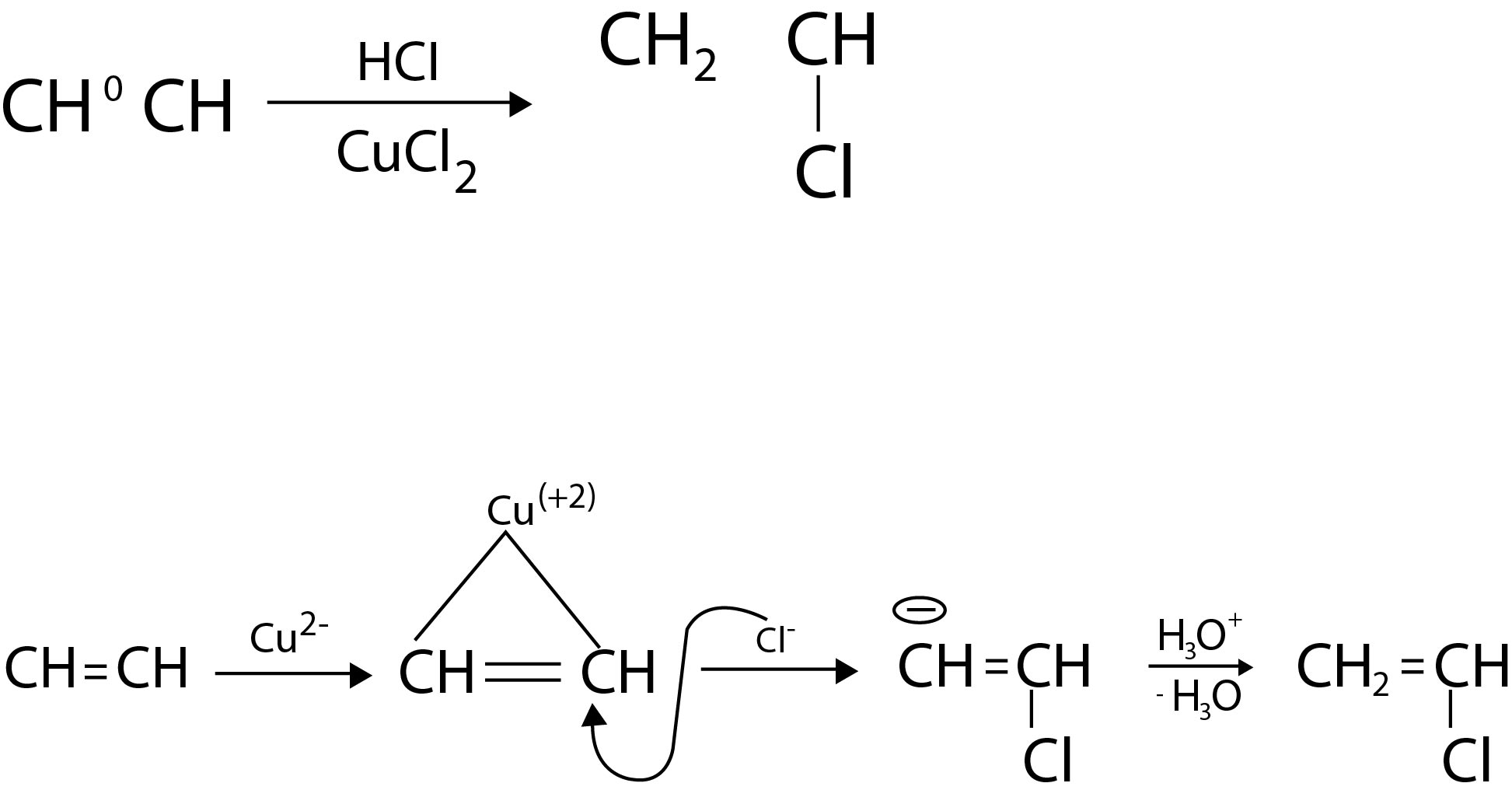
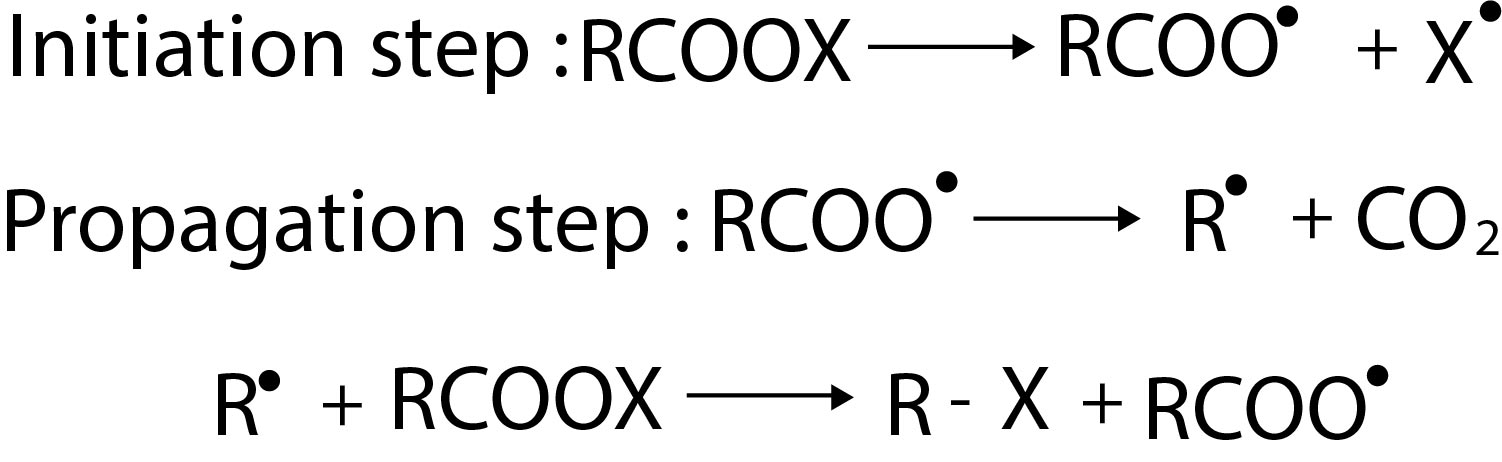From Alcohols :
(a) By using hydrogen halides
`R -OH overset(HX) rightarrow R-X+H_2O`
It must be noted that the `HX` used should be dry, which is produced in situ, as follows
`2NaCl +H_2SO_4 overset(heat) rightarrow 2HCl + Na_2SO_4`
`2NaBr + H_2SO_4 overset(heat) rightarrow 2HBr + Na_2SO_4`
`6NaI + 2H_3PO_4 overset(heat) rightarrow 6HI + 2Na_3PO_4`
It may be noted that `H_3PO_4` is used in place of `H_2SO_4` to prepare `HI`. This is because `HI` is a reducing agent and `H_2SO_4` being an oxidising agent can oxidize it.
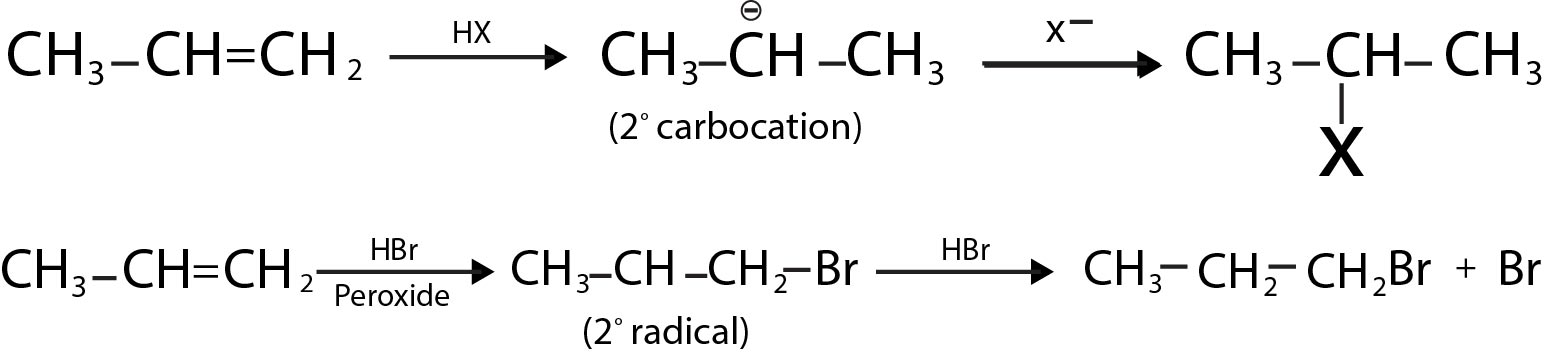
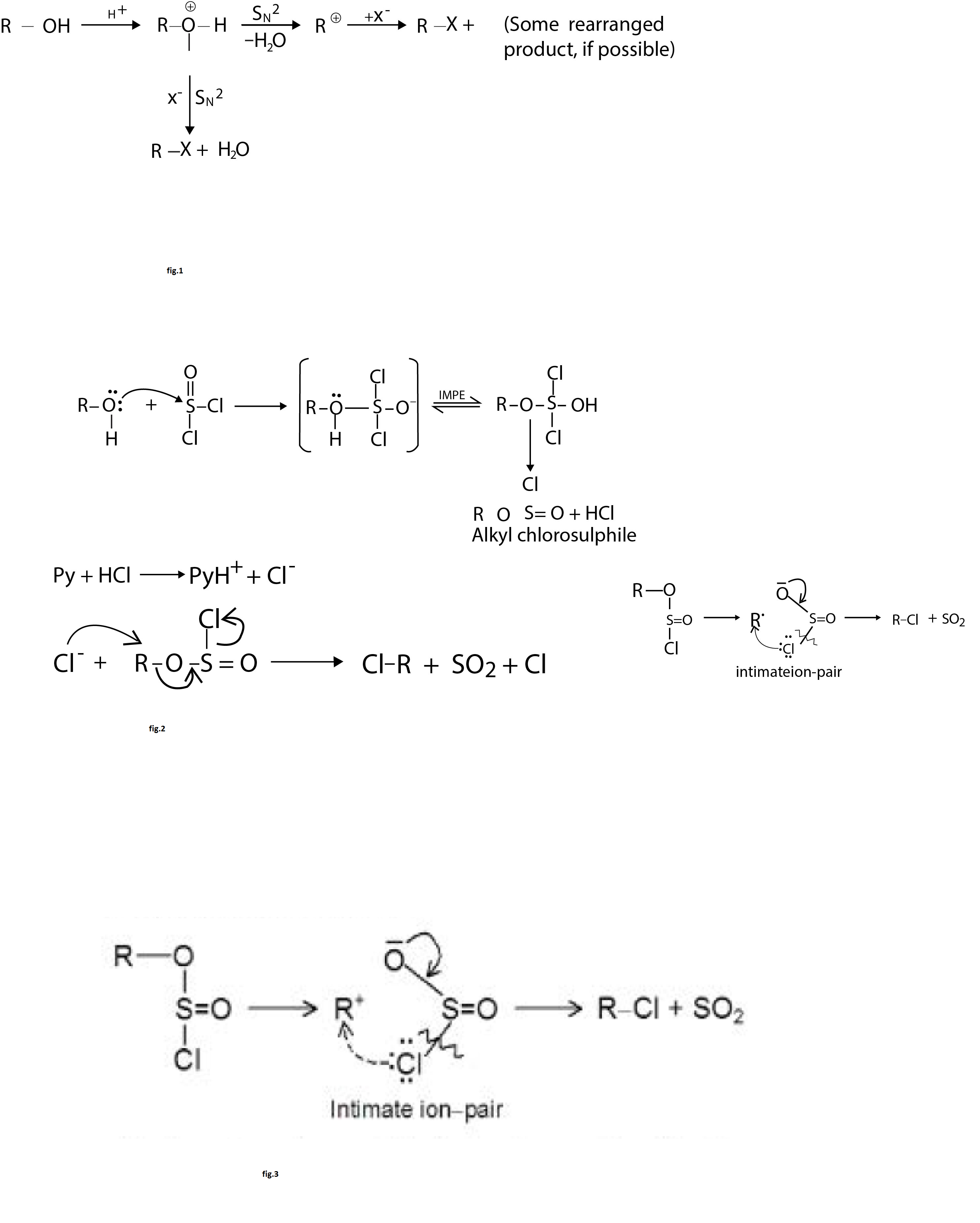
The above conversion of alcohol to alkyl halides proceeds via `S_N 1` or `S_N2` mechanism. Both the mechanisms are operative during the reaction, having competition between them. The type of mechanism followed by an alcohol depends on the structure of alcohol and the type of solvent used for carrying out reaction. See fig.1.
(b) By using phosphorous halides
`R -OH + PCl_5 rightarrow R - Cl + POCl_3 + HCl`
`3R- OH + PCl_3 rightarrow 3R -Cl + H_3PO_3`
`3R -OH + PBr_3 rightarrow 3R -Br +H_3PO_3`
`3R - OH + PI_3 rightarrow 3R -I + H_3 PO_3`
Phosphorous halides are prepared by treating red phosphorous and halogen. The advantage of using phosphorous halides is that the reaction does not involve carbocation intermediate so, it is free from rearrangement.
(c) By using `SOCl_2` (thionyl chloride)
`R - OH + SOCl_2 oversettext(Pyridine)rightarrow R -Cl + SO_2 + HCl`
The usefulness of this method is that there is no side product, which has to be separated. The side products are gaseous `SO_2`, which escape from the reaction mixture and `HCl`, which forms a salt with the base (pyridine), named pyridinium chloride (`C_5H_5N^+Cl^-` ). The product alkyl chloride has a configuration inverted with respect to the reactant alcohol (if it is chiral) in the presence of pyridine base. In absence of a base and polar solvent, the chiral alcohol gives alkyl chloride with retention of configuration.
`text(Mechanism :)` Alcohol first reacts with `SOCl_2` to form an intermediate chlorosulphite ester, which gives alkyl chlorosulphite and `HCl`. In presence of pyridine, `HCl` reacts with it to give pyridinium `(PyH ^+)` ion and chloride (`Cl^ -`) ion. The `Cl^-` displaces the leaving group `ClSO _2` and chloro sulphite ester decomposes to `SO_2, Cl^ -` and `R - Cl` with inversion of configuration. See fig.2.
In the absence of a base and polar solvent, the chlorosulphite ester dissociates into an intimate ion - pair. The `Cl` of the anion of ion- pair attacks from the front side of `R^+` to give retention of configuration. The retention is observed because `Cl` cannot reach the rear of the `R^+` group but is close to its front side. See fig.3.
This is referred as `S_N1` (substitution nucleophilic internal) mechanism because a part of the leaving group detaches itself from the rest of the leaving group during the process and attacks the substrate.
`R -OH overset(HX) rightarrow R-X+H_2O`
It must be noted that the `HX` used should be dry, which is produced in situ, as follows
`2NaCl +H_2SO_4 overset(heat) rightarrow 2HCl + Na_2SO_4`
`2NaBr + H_2SO_4 overset(heat) rightarrow 2HBr + Na_2SO_4`
`6NaI + 2H_3PO_4 overset(heat) rightarrow 6HI + 2Na_3PO_4`
It may be noted that `H_3PO_4` is used in place of `H_2SO_4` to prepare `HI`. This is because `HI` is a reducing agent and `H_2SO_4` being an oxidising agent can oxidize it.
The above conversion of alcohol to alkyl halides proceeds via `S_N 1` or `S_N2` mechanism. Both the mechanisms are operative during the reaction, having competition between them. The type of mechanism followed by an alcohol depends on the structure of alcohol and the type of solvent used for carrying out reaction. See fig.1.
(b) By using phosphorous halides
`R -OH + PCl_5 rightarrow R - Cl + POCl_3 + HCl`
`3R- OH + PCl_3 rightarrow 3R -Cl + H_3PO_3`
`3R -OH + PBr_3 rightarrow 3R -Br +H_3PO_3`
`3R - OH + PI_3 rightarrow 3R -I + H_3 PO_3`
Phosphorous halides are prepared by treating red phosphorous and halogen. The advantage of using phosphorous halides is that the reaction does not involve carbocation intermediate so, it is free from rearrangement.
(c) By using `SOCl_2` (thionyl chloride)
`R - OH + SOCl_2 oversettext(Pyridine)rightarrow R -Cl + SO_2 + HCl`
The usefulness of this method is that there is no side product, which has to be separated. The side products are gaseous `SO_2`, which escape from the reaction mixture and `HCl`, which forms a salt with the base (pyridine), named pyridinium chloride (`C_5H_5N^+Cl^-` ). The product alkyl chloride has a configuration inverted with respect to the reactant alcohol (if it is chiral) in the presence of pyridine base. In absence of a base and polar solvent, the chiral alcohol gives alkyl chloride with retention of configuration.
`text(Mechanism :)` Alcohol first reacts with `SOCl_2` to form an intermediate chlorosulphite ester, which gives alkyl chlorosulphite and `HCl`. In presence of pyridine, `HCl` reacts with it to give pyridinium `(PyH ^+)` ion and chloride (`Cl^ -`) ion. The `Cl^-` displaces the leaving group `ClSO _2` and chloro sulphite ester decomposes to `SO_2, Cl^ -` and `R - Cl` with inversion of configuration. See fig.2.
In the absence of a base and polar solvent, the chlorosulphite ester dissociates into an intimate ion - pair. The `Cl` of the anion of ion- pair attacks from the front side of `R^+` to give retention of configuration. The retention is observed because `Cl` cannot reach the rear of the `R^+` group but is close to its front side. See fig.3.
This is referred as `S_N1` (substitution nucleophilic internal) mechanism because a part of the leaving group detaches itself from the rest of the leaving group during the process and attacks the substrate.