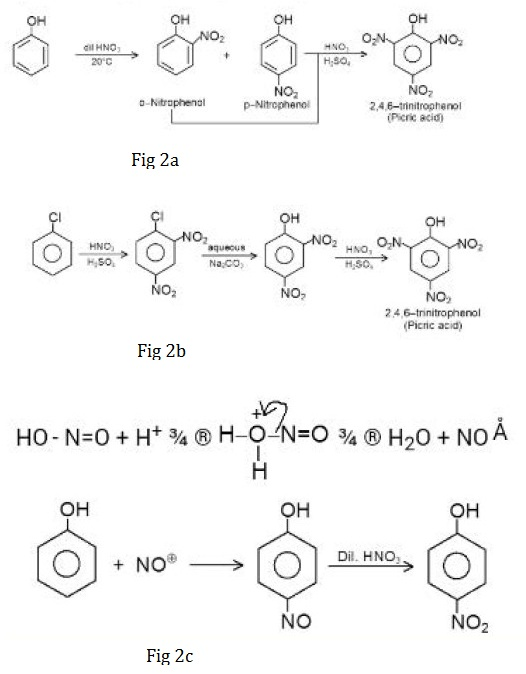
Bromination :
Phenol on treatment with chlorine or bromine water gives an immediate precipitate of `2,4,6` - trihalogen derivative. Phenol in aqueous medium is partially ionised and the phenoxide ion thus obtained is much more reactive than phenol itself towards electrophilic attack.
Moreover, halogen reacts with water forming halogen acid and hypohalous acid. The proton attacks at the `-OH` group of hypohalous acid to give `H_2O` and `- X`, which acts as a stronger electrophile. Therefore, halogenation takes place at all the ortho and para positions as shown in Fig 1a.
If the halogenation is carried out in non aqueous medium like `CS_2` or `C Cl_4`. only monosubstitution takes place as shown in Fig 1b. This is because the ionisation of phenol does not occur in non - aqueous medium. The benzene ring of phenol is less activated than that of phenoxide ion as well as the bromine in `Br_2` molecule is not as electrophilic as in `Br - overset (+) (O)H_2`.
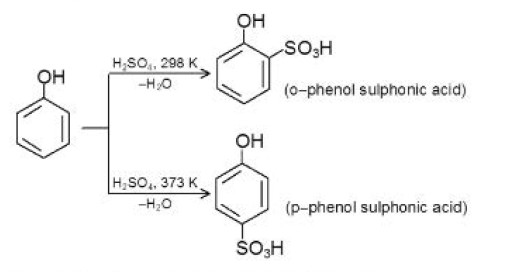
`o `- Bromophenol is also prepared (as in Fig 1c) by protecting one ortho and the para positions by sulphonation.
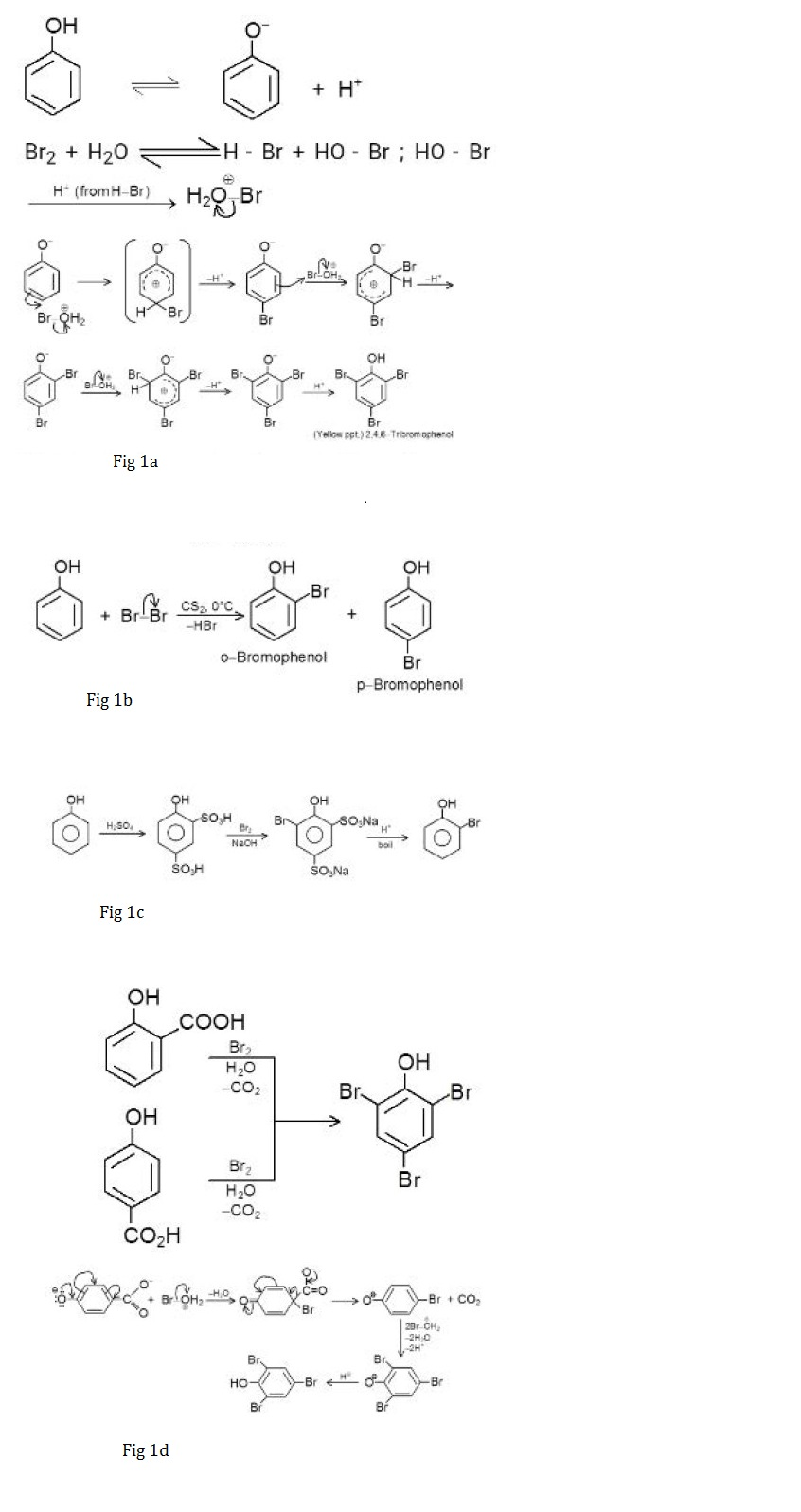
So strong is the activation of benzene ring in aqueous medium that derivatives of phenol containing - `COOH` group or- `SO_3H` group either at the ortho or at the para position are displaced by -`Br` in bromination reaction. This is an example of brominative decarboxylation. For example, salicylic acid when treated with bromine water gives `2,4,6` - trisubstituted phenol as given in Fig 1d.
In case when -`SO_3H` group is present at ortho and para positions, desulphonation takes place liberating `SO_3` gas. Trisubstitution of benzene is also observed when aniline is treated with bromine because the reactivity of aniline is same as that of phenoxide ion towards electrophilic attack.
Moreover, halogen reacts with water forming halogen acid and hypohalous acid. The proton attacks at the `-OH` group of hypohalous acid to give `H_2O` and `- X`, which acts as a stronger electrophile. Therefore, halogenation takes place at all the ortho and para positions as shown in Fig 1a.
If the halogenation is carried out in non aqueous medium like `CS_2` or `C Cl_4`. only monosubstitution takes place as shown in Fig 1b. This is because the ionisation of phenol does not occur in non - aqueous medium. The benzene ring of phenol is less activated than that of phenoxide ion as well as the bromine in `Br_2` molecule is not as electrophilic as in `Br - overset (+) (O)H_2`.
`o `- Bromophenol is also prepared (as in Fig 1c) by protecting one ortho and the para positions by sulphonation.
So strong is the activation of benzene ring in aqueous medium that derivatives of phenol containing - `COOH` group or- `SO_3H` group either at the ortho or at the para position are displaced by -`Br` in bromination reaction. This is an example of brominative decarboxylation. For example, salicylic acid when treated with bromine water gives `2,4,6` - trisubstituted phenol as given in Fig 1d.
In case when -`SO_3H` group is present at ortho and para positions, desulphonation takes place liberating `SO_3` gas. Trisubstitution of benzene is also observed when aniline is treated with bromine because the reactivity of aniline is same as that of phenoxide ion towards electrophilic attack.