Introduction :
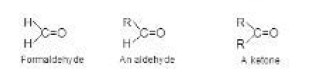
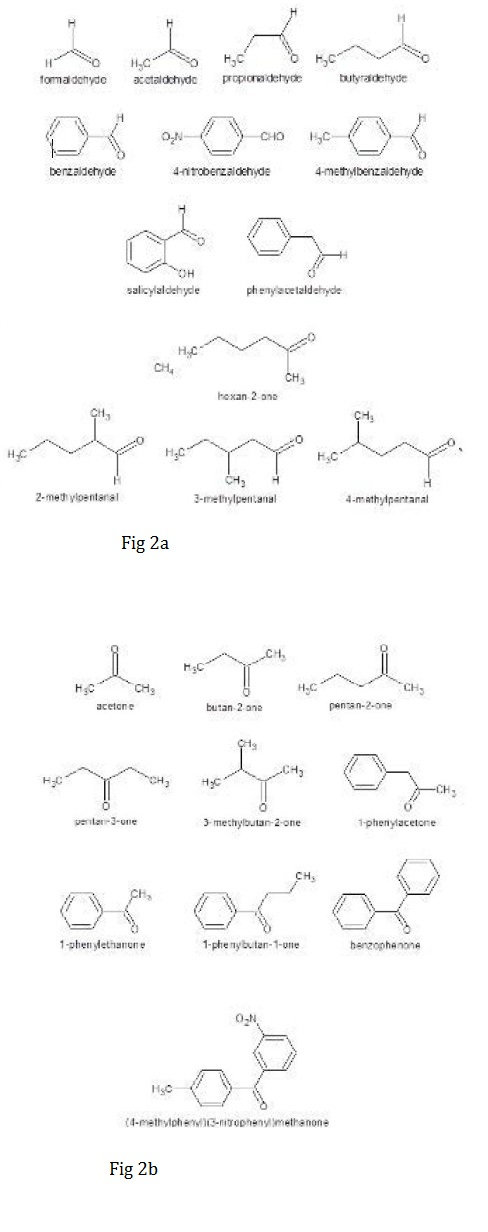
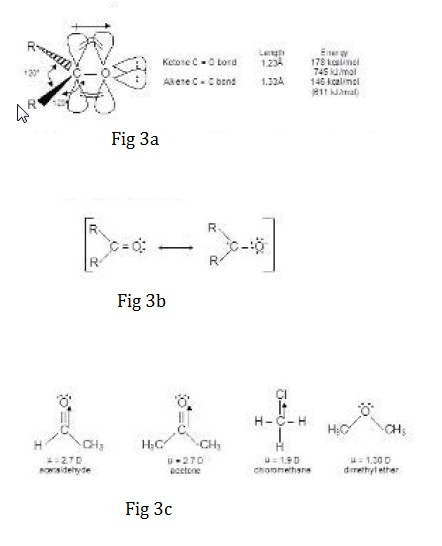
Aldehydes and ketones both possess a carbonyl group `( rangle C=O)` and therefore are called carbonyl compounds. Formaldehyde is the simplest aldehyde, bonded to two hydrogens. In all other aldehydes, the carbonyl group is bonded to a hydrogen and to an alkyl (or an aryl) group. The carbonyl group of a ketone is bonded to two alkyl (or aryl) groups as shown in fig 1.
Aldehydes and ketones undergo two types of characteristic reactions.
(a) Nucleophilic addition reactions :
The carbonyl compounds can be readily attacked by nucleophiles due to the presence of electrophilic carbon. In the overall reaction, nucleophilic addition takes place and not the nucleophilic substitution as aldehydes and ketones possess very poor leaving groups, `R`-and `H`- respectively.
(b) Reactions due to acidity of `alpha`-hydrogen atoms :
Carbonyl compounds with `alpha`-hydrogen atoms can loose `H^+` to a base to give carbanion. which is resonance stabilized. This carbanion acts as nucleophile and can add to the electrophilic carbon of same or different carbonyl compound to give final product.
Aldehydes and ketones undergo two types of characteristic reactions.
(a) Nucleophilic addition reactions :
The carbonyl compounds can be readily attacked by nucleophiles due to the presence of electrophilic carbon. In the overall reaction, nucleophilic addition takes place and not the nucleophilic substitution as aldehydes and ketones possess very poor leaving groups, `R`-and `H`- respectively.
(b) Reactions due to acidity of `alpha`-hydrogen atoms :
Carbonyl compounds with `alpha`-hydrogen atoms can loose `H^+` to a base to give carbanion. which is resonance stabilized. This carbanion acts as nucleophile and can add to the electrophilic carbon of same or different carbonyl compound to give final product.