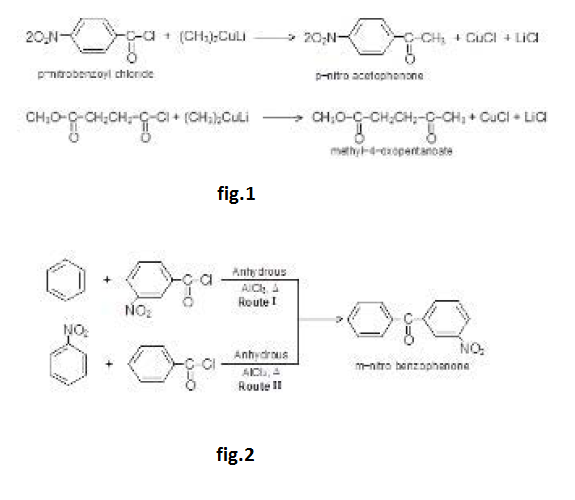
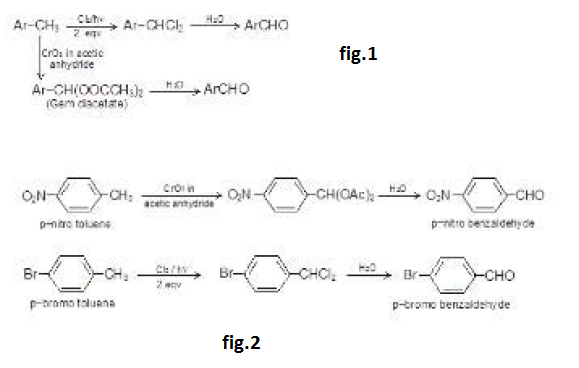
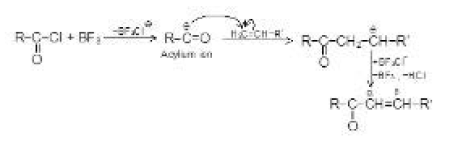
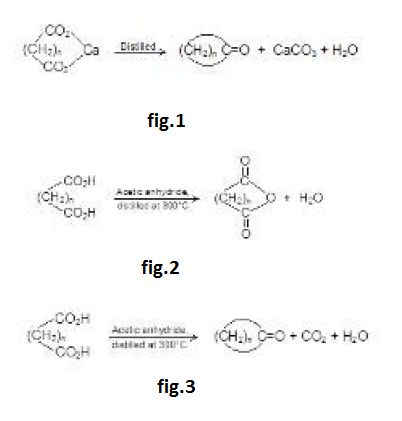
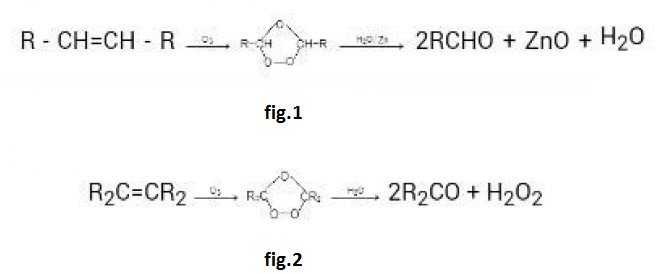
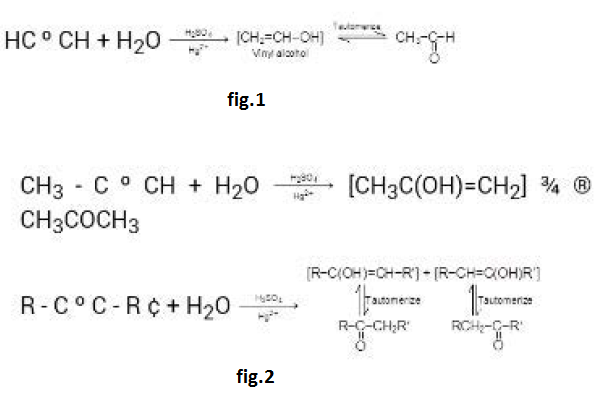
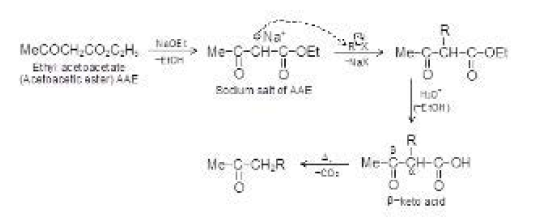
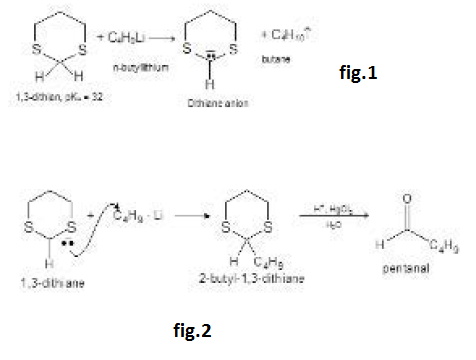
By Oxidation of Alcohols :
The oxidation of an alcohol involves the loss of one or more `alpha`-hydrogen atoms from the carbon bearing `-OH` group. The kind of product formed depends upon how many of these `alpha`-hydrogen atoms the alcohol contains. i.e. whether the alcohol is primary or secondary.
A primary alcohol (which has 2 `alpha`-hydrogens) can lose one of the hydrogen to form an aldehyde whereas secondary alcohol can lose its only `alpha` -hydrogen to form a ketone.
`RCH_2 OH underset(KMnO_4 text(Aqueous acidic)K_2Cr_2O_7)oversettext(Aqueous acidic)(->) RCHO + MnO_2` or `Cr^(3+)`
The reagents employed here are not highly selective since aldehyde can further be easily oxidised to carboxylic acids, so isolation of aldehydes in this reaction is troublesome. Thus, for the convenient and selective conversion of primary alcohol to aldehyde, pyridinium chlorochromate(PC C, `C_5H_5NH^+CrO_3Cl^-`) is used, which is formed by the reaction between chromic acid and pyridinium chloride.
`RCH_2OH underset[text(or) CrO_2 text(in glacial acidic acid or) CrO_3 text(in pyridine)]overset[C_2H_5overset(oplus)NHCrO_2overset(⊖)Cl(PC C)]-> RCHO +Cr^(3+)`
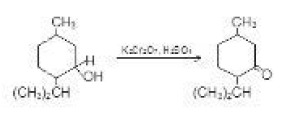
`R_2CHOH underset(text(or Aqueous acidic) K_2Cr_2O_7 text(or) CrO_2 text(in glacial acidic acid or) CrO_3 text(in pyridine))overset[text(Aqueous acidic) KMnO_4]-> R_2 C +MnO_2 `or `Cr^(3+)`
Secondary alcohols can also be oxidised by aluminium `t`- butoxide, `[(CH_3)_3CO]_3Al` in acetone. The reaction is called Oppenauer oxidation. In presence of `p` -benzoquinone solvent `1^o` alcohol can also be oxidised to aldehyde on distillation.
`R_2CHOH undersettext(in acetone)overset([(CH_3)_3CO]_3Al)-> R_2 CO + (CH_3 )_2 CHOH`
For example,
`CH_3CH_2CH_2OH underset(CH_2Cl_2)overset[PC C text(in)]-> CH_3CH_2CHO + Cr^(3+)`
See fig.
A primary alcohol (which has 2 `alpha`-hydrogens) can lose one of the hydrogen to form an aldehyde whereas secondary alcohol can lose its only `alpha` -hydrogen to form a ketone.
`RCH_2 OH underset(KMnO_4 text(Aqueous acidic)K_2Cr_2O_7)oversettext(Aqueous acidic)(->) RCHO + MnO_2` or `Cr^(3+)`
The reagents employed here are not highly selective since aldehyde can further be easily oxidised to carboxylic acids, so isolation of aldehydes in this reaction is troublesome. Thus, for the convenient and selective conversion of primary alcohol to aldehyde, pyridinium chlorochromate(PC C, `C_5H_5NH^+CrO_3Cl^-`) is used, which is formed by the reaction between chromic acid and pyridinium chloride.
`RCH_2OH underset[text(or) CrO_2 text(in glacial acidic acid or) CrO_3 text(in pyridine)]overset[C_2H_5overset(oplus)NHCrO_2overset(⊖)Cl(PC C)]-> RCHO +Cr^(3+)`
`R_2CHOH underset(text(or Aqueous acidic) K_2Cr_2O_7 text(or) CrO_2 text(in glacial acidic acid or) CrO_3 text(in pyridine))overset[text(Aqueous acidic) KMnO_4]-> R_2 C +MnO_2 `or `Cr^(3+)`
Secondary alcohols can also be oxidised by aluminium `t`- butoxide, `[(CH_3)_3CO]_3Al` in acetone. The reaction is called Oppenauer oxidation. In presence of `p` -benzoquinone solvent `1^o` alcohol can also be oxidised to aldehyde on distillation.
`R_2CHOH undersettext(in acetone)overset([(CH_3)_3CO]_3Al)-> R_2 CO + (CH_3 )_2 CHOH`
For example,
`CH_3CH_2CH_2OH underset(CH_2Cl_2)overset[PC C text(in)]-> CH_3CH_2CHO + Cr^(3+)`
See fig.