Physical Properties :
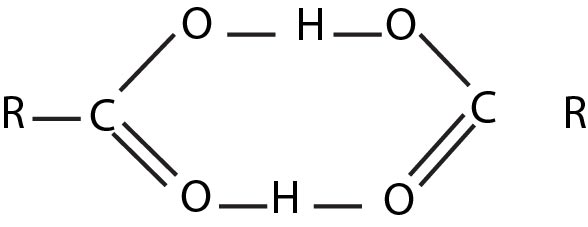
The molecules of carboxylic acids are polar and exhibit hydrogen bonding. The first four are miscible with water. The higher acids are virtually insoluble. The simplest aromatic acid, benzoic acid, contains too many carbon atoms to show appreciable solubility in water.
Carboxylic acids are soluble in less polar solvents like ether, alcohol, benzene etc.
Carboxylic acids have higher boiling points than alcohols. These very high boiling points are due to the fact that a pair of carboxylic acid molecules are held together not by one but by two hydrogen bonds and exist as dimer.
A study of nitrated spectra of formic acid in the liquid and solid states has provided evidence that this acid, unlike most of the other carboxylic acids, is not dimeric in these states, but is associated as a polymer.
The first three fatty acids are colourless pungent smelling liquids.
Carboxylic acids are soluble in less polar solvents like ether, alcohol, benzene etc.
Carboxylic acids have higher boiling points than alcohols. These very high boiling points are due to the fact that a pair of carboxylic acid molecules are held together not by one but by two hydrogen bonds and exist as dimer.
A study of nitrated spectra of formic acid in the liquid and solid states has provided evidence that this acid, unlike most of the other carboxylic acids, is not dimeric in these states, but is associated as a polymer.
The first three fatty acids are colourless pungent smelling liquids.