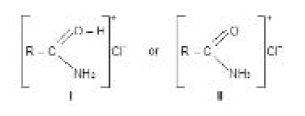
Hydrolysis of Amides :
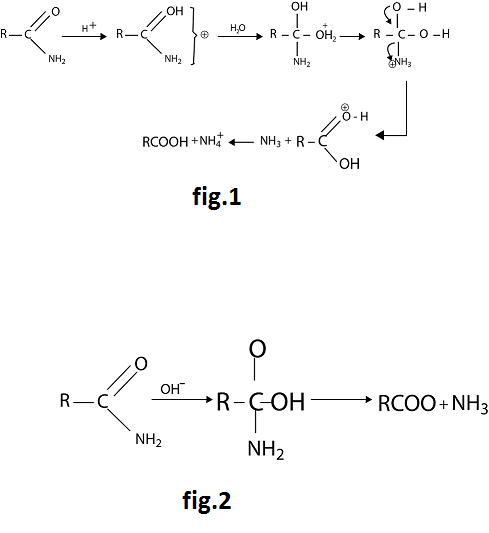
It involves nucleophilic substitution, in which the `NH_2` group is replaced by `- OH`. Under acidic conditions hydrolysis involves attack by water on the protonated amide. See fig.1.
Under alkaline conditions hydrolysis involves attack by the strongly nucleophilic hydroxide ion on the amide itself. See fig.2.
Under alkaline conditions hydrolysis involves attack by the strongly nucleophilic hydroxide ion on the amide itself. See fig.2.