Surface Energy
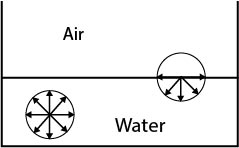
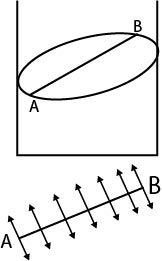
The molecules on the surface experience a net downward force. Therefore, to bring a molecule from interior of liquid to the surface. some work is required to be done against inter molecular forces.
This work is stored as potential energy of the molecule on the surface.
The potential energy of surface molecules per unit area of the surface is called surface energy.
Its `S.l` unit is `J//m^2` and dimensions are `[M^1L^2T^2]`
It depends on the number of surfaces present. For example, a liquid drop has one liquid-air surface while bubble has two liquid-air surfaces.
`text(Measurement of surface energy)`
Consider a rectangular wire frame ABCD with sliding armAB.
When this frame is dipped in a soap solution. a soap film is formed in frame.
Due to surface tension (T), the film exerts a force on the frame (AB) towards interior of film is given by
`F= T xx (2l)` (Two surface of film)
If arm AB is displaced to new position A'B' through distance (dx) then, work done ( dW) is given by
`dw = F dx = 2Tl(dx)`
`W = int dW = int 2Tldx = 2Tlx`
`W = TA`
Where. `A = 2lx =` Area of both surfaces of film
The work done to form a film is stored as potential energy of the surface. The amount of work done or potential energy per unit area under isothermal conditions is equal to surface energy.
Surface energy `= W/A `
Surface energy is numerically equal to surface tension.
This work is stored as potential energy of the molecule on the surface.
The potential energy of surface molecules per unit area of the surface is called surface energy.
Its `S.l` unit is `J//m^2` and dimensions are `[M^1L^2T^2]`
It depends on the number of surfaces present. For example, a liquid drop has one liquid-air surface while bubble has two liquid-air surfaces.
`text(Measurement of surface energy)`
Consider a rectangular wire frame ABCD with sliding armAB.
When this frame is dipped in a soap solution. a soap film is formed in frame.
Due to surface tension (T), the film exerts a force on the frame (AB) towards interior of film is given by
`F= T xx (2l)` (Two surface of film)
If arm AB is displaced to new position A'B' through distance (dx) then, work done ( dW) is given by
`dw = F dx = 2Tl(dx)`
`W = int dW = int 2Tldx = 2Tlx`
`W = TA`
Where. `A = 2lx =` Area of both surfaces of film
The work done to form a film is stored as potential energy of the surface. The amount of work done or potential energy per unit area under isothermal conditions is equal to surface energy.
Surface energy `= W/A `
Surface energy is numerically equal to surface tension.