The Breaking and Forming of Bonds :
A covalent bond between two atoms can be broken in essentially the following ways: fig-1.
In the first case each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron; this is referred to as homolytic fission of the bond. Alternatively, one atom may hold on to both electrons, leaving none for the other, the result in the above case being a negative and positive ion, respectively. Where `R` and `X` are not identical, the fission, can, of course, take place in either of two ways, as shown above, depending on whether `R` or `X` retains the electron pair. Either of these processes is referred to as heterolytic fission, the result being the formation of an ion pair. Formation of a covalent bond can take place by the reversal of any of these processes, and also, of course, by the attack of first formed radicals or ions on other species :
`R^* + Br - Br -> R - Br +Br^*`
`RA +H_2O -> R-OH + HA`
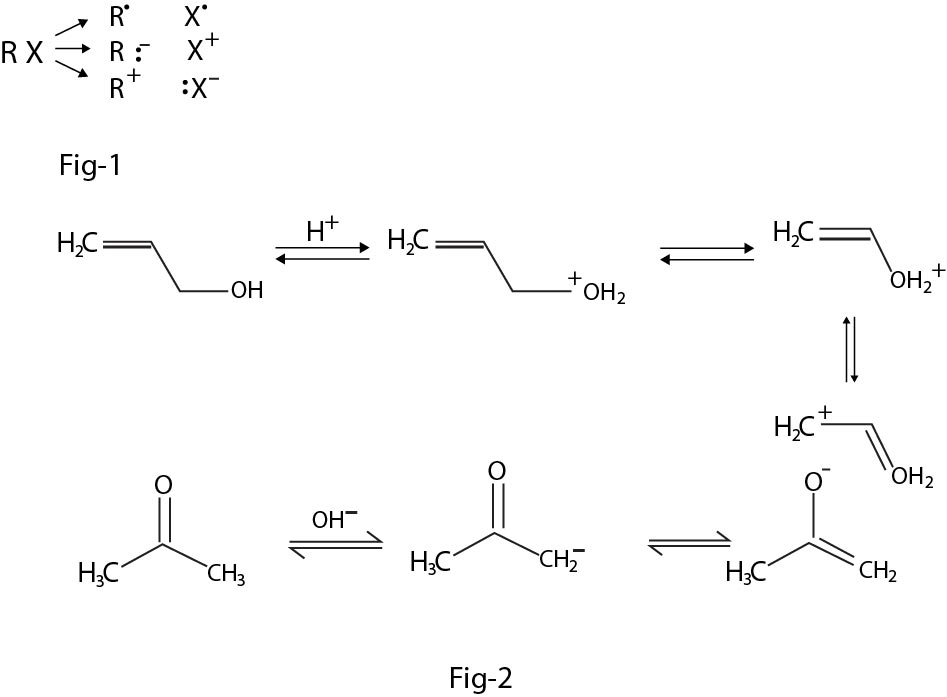
Such radicals or ion pairs are formed transiently as reactive intermediates in a very wide variety of organic reactions, as will be shown below. Reactions involving radicals tend to occur in the gas phase and in solution in non-polar solvents, and to be catalysed by light and by the addition of other radicals. Reactions involving ionic intermediates take place more readily in solution in polar solvents, because of the greater ease of separation of charges therein and very often because of the stabilisation of the resultant ion pairs through solvation. Many of these ionic intermediates can be considered as carrying their charge on a carbon atom, though the ion is often stabilised by delocalisation of the charge, to a greater or lesser extent, over other carbon atoms, or atoms of different elements : See fig-2.
When a positive charge is carried on carbon the entity, is known as a carbocation, and when a negative charge, a carbanion. Though such ions may be formed only transiently and be present only in minute concentration, they are nevertheless often of paramount importance in controlling the reactions in which they participate.
These three types, radicals, carbocations and carbanions, by no means exhaust the possibilities of transient intermediates in which carbon is the active centre : others include the electron-deficient species carbenes.
In the first case each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron; this is referred to as homolytic fission of the bond. Alternatively, one atom may hold on to both electrons, leaving none for the other, the result in the above case being a negative and positive ion, respectively. Where `R` and `X` are not identical, the fission, can, of course, take place in either of two ways, as shown above, depending on whether `R` or `X` retains the electron pair. Either of these processes is referred to as heterolytic fission, the result being the formation of an ion pair. Formation of a covalent bond can take place by the reversal of any of these processes, and also, of course, by the attack of first formed radicals or ions on other species :
`R^* + Br - Br -> R - Br +Br^*`
`RA +H_2O -> R-OH + HA`
Such radicals or ion pairs are formed transiently as reactive intermediates in a very wide variety of organic reactions, as will be shown below. Reactions involving radicals tend to occur in the gas phase and in solution in non-polar solvents, and to be catalysed by light and by the addition of other radicals. Reactions involving ionic intermediates take place more readily in solution in polar solvents, because of the greater ease of separation of charges therein and very often because of the stabilisation of the resultant ion pairs through solvation. Many of these ionic intermediates can be considered as carrying their charge on a carbon atom, though the ion is often stabilised by delocalisation of the charge, to a greater or lesser extent, over other carbon atoms, or atoms of different elements : See fig-2.
When a positive charge is carried on carbon the entity, is known as a carbocation, and when a negative charge, a carbanion. Though such ions may be formed only transiently and be present only in minute concentration, they are nevertheless often of paramount importance in controlling the reactions in which they participate.
These three types, radicals, carbocations and carbanions, by no means exhaust the possibilities of transient intermediates in which carbon is the active centre : others include the electron-deficient species carbenes.