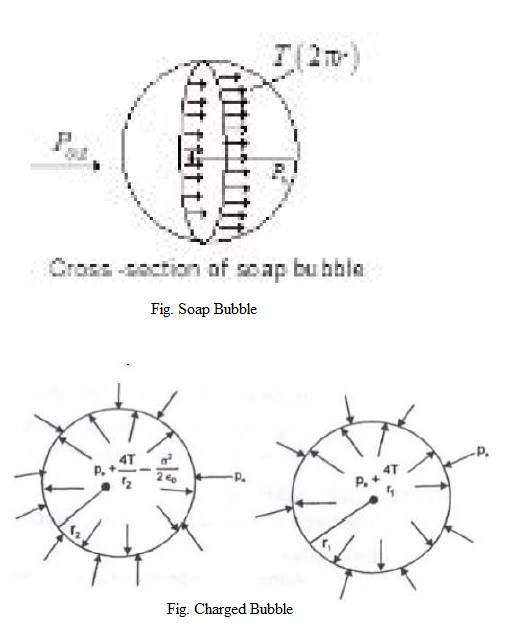
Capillarity
It is a phenomenon by which a glass tube of very fine bore (called capillary tube) is dipped in a liquid (say water), the liquid immediately rises up due to surface tension.
The narrower the tube, the greater is the height to which liquid rises.
When the same capillary tube is placed in mercury, then it gets depressed below the outside level.
The depression increases as the diameter of the tube decreases.
Liquids which rise in capillary are called wetting liquids and which fall are called non-wetting liquids.
Adhesive force is the attractive force between molecules of two different materials.
Cohesive force is the attractive force among molecules of same material.
`text(Measurement of capillary Rise :)`
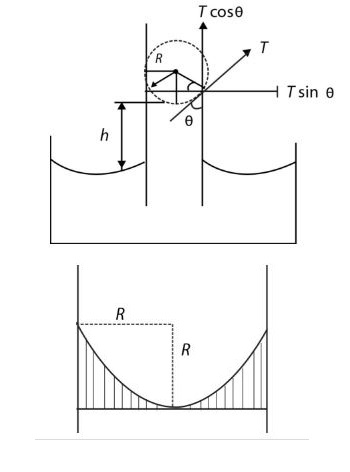
Consider a glass capillary tube of radius (R) dipped vertically in liquid as shown.
The liquid meets the tube at angle of contact `(theta)`
The water meniscus in tube is along a circle of circumference , `2 pi r` which is in contact with glass.
Due to surface tension, a force equal to (T) per unit length acts at all points of the circle (Meniscus) on tube.
If `(theta)` is angle of contact, then this force is inwards at an angle `(theta)` from the wall on tube.
From Newton's third law tube exerts an equal and opposite force `T` per unit length on the circumference of the liquid meniscus.
Resolving the force into two components
`T cos theta =` Force per unit length on liquid acting vertically upward
`T sin theta =` Force per unit length on liquid acting horizontally outward
Considering the entire circumference `(2 pi r)` for each horizontal component `r sin theta` there is equal and diametrically opposite component and they neutralize each other.
Hence, net force in horizontal direction = 0
The vertical components being in same direction are added up and give net upward force `(2 pi r)(T cos theta)`
This force supports the weight of water column `(2 pi r)(T cos theta) =` Weight of the liquid column
`= (pi r^2)(h)(rho g) + V rho g`
Where, V = volume of liquid in meniscus,
`h= (2T cos theta)/(R rho g)`
If volume of liquid in meniscus is negligible.
`NOTE :`
i. The small volume of liquid in meniscus (V) can be calculated as
For pure water and glass
`theta = 0^o`
So, the meniscus is hemi-spherical
V = volume of cylinder of height (R) - volume of Hemisphere of radius (R)
`V = (pi R^2)- 1/2 ((4 pi)/3 R^2),` `V = pi R^2 - (2 pi)/3 R^3 = 1/3 pi R^3`
For water and glass
`(T cos0^@)(2 pi R) = (pi R^2h rho g) = 1/3 pi R^3 rho g ,` `h= (2T)/(R rho g) - (R/3)`
ii. If angle of contact `(theta)` is greater than goo, the term `(cos theta)` is negative and hence h is negative. The expression gives the depression of liquid in the tube.
iii. R = radius of tube
r =radius of meniscus
`cos theta = (R/r)` or `r =(R /cos theta)` , `h = (2T cos theta)/(R rho g) = (2T)/(r rho g)`
iv. If the length of tube is greater than (h), the liquid rises in the tube so as to satisfy the above relation. But if the length of tube is insufficient, then angle made by liquid surface `(theta)` and hence radius of meniscus (r) changes in such a way that the force
` (2 pi R) T cos theta =` Weight to liquid rise
`= (h^2 rho g)pi R^2`
`h= (2T cos theta')/(r rho g) = (2T)/(r' rho g)` Or `h'r' = (2T)/(rho g) =` Constant `= hr`
`h'r' = hr =` constant
The narrower the tube, the greater is the height to which liquid rises.
When the same capillary tube is placed in mercury, then it gets depressed below the outside level.
The depression increases as the diameter of the tube decreases.
Liquids which rise in capillary are called wetting liquids and which fall are called non-wetting liquids.
Adhesive force is the attractive force between molecules of two different materials.
Cohesive force is the attractive force among molecules of same material.
`text(Measurement of capillary Rise :)`
Consider a glass capillary tube of radius (R) dipped vertically in liquid as shown.
The liquid meets the tube at angle of contact `(theta)`
The water meniscus in tube is along a circle of circumference , `2 pi r` which is in contact with glass.
Due to surface tension, a force equal to (T) per unit length acts at all points of the circle (Meniscus) on tube.
If `(theta)` is angle of contact, then this force is inwards at an angle `(theta)` from the wall on tube.
From Newton's third law tube exerts an equal and opposite force `T` per unit length on the circumference of the liquid meniscus.
Resolving the force into two components
`T cos theta =` Force per unit length on liquid acting vertically upward
`T sin theta =` Force per unit length on liquid acting horizontally outward
Considering the entire circumference `(2 pi r)` for each horizontal component `r sin theta` there is equal and diametrically opposite component and they neutralize each other.
Hence, net force in horizontal direction = 0
The vertical components being in same direction are added up and give net upward force `(2 pi r)(T cos theta)`
This force supports the weight of water column `(2 pi r)(T cos theta) =` Weight of the liquid column
`= (pi r^2)(h)(rho g) + V rho g`
Where, V = volume of liquid in meniscus,
`h= (2T cos theta)/(R rho g)`
If volume of liquid in meniscus is negligible.
`NOTE :`
i. The small volume of liquid in meniscus (V) can be calculated as
For pure water and glass
`theta = 0^o`
So, the meniscus is hemi-spherical
V = volume of cylinder of height (R) - volume of Hemisphere of radius (R)
`V = (pi R^2)- 1/2 ((4 pi)/3 R^2),` `V = pi R^2 - (2 pi)/3 R^3 = 1/3 pi R^3`
For water and glass
`(T cos0^@)(2 pi R) = (pi R^2h rho g) = 1/3 pi R^3 rho g ,` `h= (2T)/(R rho g) - (R/3)`
ii. If angle of contact `(theta)` is greater than goo, the term `(cos theta)` is negative and hence h is negative. The expression gives the depression of liquid in the tube.
iii. R = radius of tube
r =radius of meniscus
`cos theta = (R/r)` or `r =(R /cos theta)` , `h = (2T cos theta)/(R rho g) = (2T)/(r rho g)`
iv. If the length of tube is greater than (h), the liquid rises in the tube so as to satisfy the above relation. But if the length of tube is insufficient, then angle made by liquid surface `(theta)` and hence radius of meniscus (r) changes in such a way that the force
` (2 pi R) T cos theta =` Weight to liquid rise
`= (h^2 rho g)pi R^2`
`h= (2T cos theta')/(r rho g) = (2T)/(r' rho g)` Or `h'r' = (2T)/(rho g) =` Constant `= hr`
`h'r' = hr =` constant