Structure and Nomenclature of Ethers :
General formula : `R- O-R` are the same or different alkyl, aryl, alkeny, vinyl, alkynl groups.
A.) i) simple/symmetrical ethers : `R` & `R'` are the same group
e.g. `CH_3 - O - CH_3` Dimethyl ether
ii) Mixed/Unsymmetrical ethers: `R` & `R'` are different group
e.g. `C_5H_5-O-CH_2C_6H_5` Benzyl phenyl ether
B.) i) aliphatic ethers : `R` & `R'` are alkyl groups
e.g. `CH_3-O - CH_2CH_3` Ethyl methyl ether
ii) Aromatic ethers : Either are both `R` & `R'` are aryl groups
e.g. `C_6H_5-O- C_6H_5` Diphenyl ethers
Aromatic ethers are further subdivided into:
a) Phenolic ethers: One of the groups are aryl while other is alkyl or Alkyl aryl ethers
e.g. `C_6H_5- O - CH_3` Methyl phenyl ether
b) Diaryl ethers: both groups are aryl.
e.g. `C_6H_5- O- C_6H_5` Diphenyl ether
C.) There are various types of cyclic ethers also.
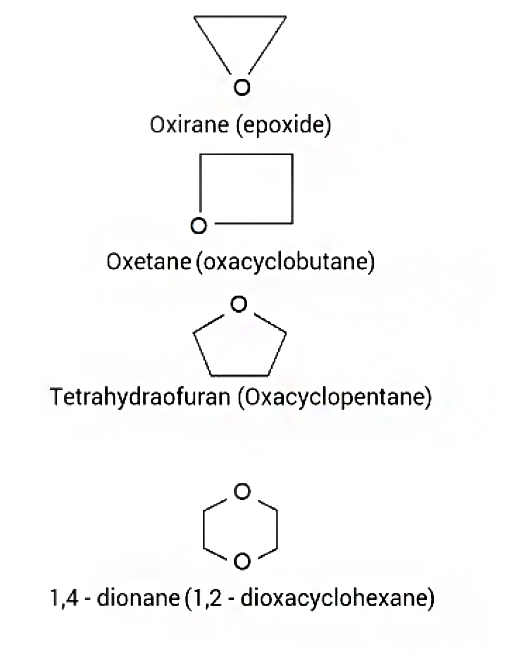
i) Cyclic ethers consisting of `2` C's in a `3` member ether are called as oxirane or Epoxides
ii) `3` C's in a `4` member ether are called oxetanes
iii) `4` C's in a `5` member ether are called tetrahydrofurans.
A.) i) simple/symmetrical ethers : `R` & `R'` are the same group
e.g. `CH_3 - O - CH_3` Dimethyl ether
ii) Mixed/Unsymmetrical ethers: `R` & `R'` are different group
e.g. `C_5H_5-O-CH_2C_6H_5` Benzyl phenyl ether
B.) i) aliphatic ethers : `R` & `R'` are alkyl groups
e.g. `CH_3-O - CH_2CH_3` Ethyl methyl ether
ii) Aromatic ethers : Either are both `R` & `R'` are aryl groups
e.g. `C_6H_5-O- C_6H_5` Diphenyl ethers
Aromatic ethers are further subdivided into:
a) Phenolic ethers: One of the groups are aryl while other is alkyl or Alkyl aryl ethers
e.g. `C_6H_5- O - CH_3` Methyl phenyl ether
b) Diaryl ethers: both groups are aryl.
e.g. `C_6H_5- O- C_6H_5` Diphenyl ether
C.) There are various types of cyclic ethers also.
i) Cyclic ethers consisting of `2` C's in a `3` member ether are called as oxirane or Epoxides
ii) `3` C's in a `4` member ether are called oxetanes
iii) `4` C's in a `5` member ether are called tetrahydrofurans.