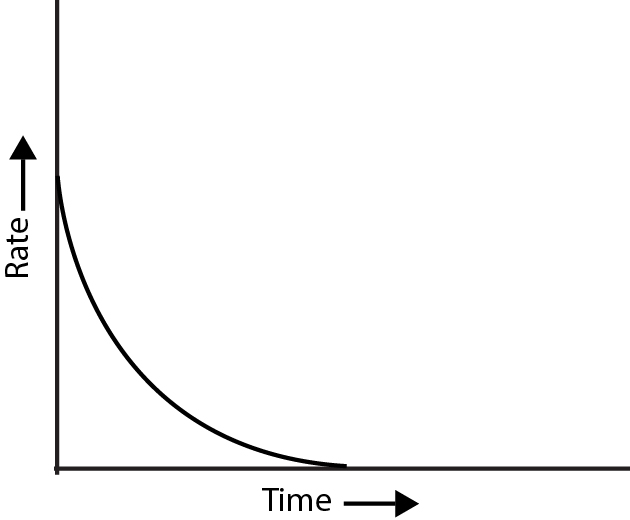
Introduction :
Chemical Kinetics is the branch of science that deals with rate of reaction, factors affecting the rate of reaction and reaction mechanism.
Different reactions occur at different rate. In fact a chemical reaction involves redistribution of bonds-breaking of bond(s) in the reactant molecule(s) and making of bonds in the product molecule(s). The rate of a chemical reaction actually depends upon the strength of the bond(s) and number of bonds to be broken during the reaction. It takes longer time for the reactant molecules to acquire higher amount of energy which they do by collision. Hence reactions involving breaking of strong bond at relatively slower rate while those involving breaking of weak bond at relatively faster rate.
On the basis of rate, reactions are classified as :
i) Instantaneous or extremely fast reactions i.e. reactions with half-life of the order of fraction of second.
ii) Extremely slow reactions i.e. reactions with half-life of the order of years.
iii) Reactions of moderate or measurable rate.
Ionic reactions are instantaneous. If a drop of silver nitrate solution is added to a solution of the chloride salt of any metal or solution of `HCl`, a white precipitate of silver chloride appears within twinkling of eye. This is because of the fact that in aqueous solution an ionic
compound exists as its constituent ions. No bond needs to be broken during the reaction. Hence reaction takes no time to complete.
For example `Na^+ + Cl^(-) + Ag^+ +NO_3^(-) ->AgCl + Na^(+) + NO_3^(-)`
There are some molecular reactions which are known to be extremely slow. Their half-lives are of the order of several years.
For example `4Fe + xH_2O+ 3O_2-> 2Fe_2O_3 * xH_2O`
Note that the reaction given above is called "rusting of iron". The half-life of this reaction is in years.
Most molecular reactions especially organic reactions occur at measurable rate. The half life of such reactions are of the order of
minutes, hours, days. Examples of such reactions are numerous. Some of these are given below.
`CH_3COOC_2H_5 + H_2O overset(H^+)-> CH_3COOH+ C_2H_5OH`
`underset [text(sucrose)](C_12H_22O_11)+H_2O overset(H^+)-> undersettext(Glucose)(C_6H_12O_6)+undersettext(Fructose)(C_6H_12O_6)`
`H_2O_2 (aq) -> 2H_2O+1/2 O_2 uparrow`
`2N_2O_5 -> 4NO_2 + O_2 uparrow`
`NH_4NO_2(aq)->2H_2O+N_2 uparrow`
In Chemical Kinetics we deal with the rates of only those reactions which occur with measurable rate i.e. which are neither too fast nor too slow. These days rates of fast reactions are also determined using lasers.
Different reactions occur at different rate. In fact a chemical reaction involves redistribution of bonds-breaking of bond(s) in the reactant molecule(s) and making of bonds in the product molecule(s). The rate of a chemical reaction actually depends upon the strength of the bond(s) and number of bonds to be broken during the reaction. It takes longer time for the reactant molecules to acquire higher amount of energy which they do by collision. Hence reactions involving breaking of strong bond at relatively slower rate while those involving breaking of weak bond at relatively faster rate.
On the basis of rate, reactions are classified as :
i) Instantaneous or extremely fast reactions i.e. reactions with half-life of the order of fraction of second.
ii) Extremely slow reactions i.e. reactions with half-life of the order of years.
iii) Reactions of moderate or measurable rate.
Ionic reactions are instantaneous. If a drop of silver nitrate solution is added to a solution of the chloride salt of any metal or solution of `HCl`, a white precipitate of silver chloride appears within twinkling of eye. This is because of the fact that in aqueous solution an ionic
compound exists as its constituent ions. No bond needs to be broken during the reaction. Hence reaction takes no time to complete.
For example `Na^+ + Cl^(-) + Ag^+ +NO_3^(-) ->AgCl + Na^(+) + NO_3^(-)`
There are some molecular reactions which are known to be extremely slow. Their half-lives are of the order of several years.
For example `4Fe + xH_2O+ 3O_2-> 2Fe_2O_3 * xH_2O`
Note that the reaction given above is called "rusting of iron". The half-life of this reaction is in years.
Most molecular reactions especially organic reactions occur at measurable rate. The half life of such reactions are of the order of
minutes, hours, days. Examples of such reactions are numerous. Some of these are given below.
`CH_3COOC_2H_5 + H_2O overset(H^+)-> CH_3COOH+ C_2H_5OH`
`underset [text(sucrose)](C_12H_22O_11)+H_2O overset(H^+)-> undersettext(Glucose)(C_6H_12O_6)+undersettext(Fructose)(C_6H_12O_6)`
`H_2O_2 (aq) -> 2H_2O+1/2 O_2 uparrow`
`2N_2O_5 -> 4NO_2 + O_2 uparrow`
`NH_4NO_2(aq)->2H_2O+N_2 uparrow`
In Chemical Kinetics we deal with the rates of only those reactions which occur with measurable rate i.e. which are neither too fast nor too slow. These days rates of fast reactions are also determined using lasers.