Radio Active Decay Law
Rate of disintegration of the radioactive substance, at any instant, is directly proportional to the number of atoms present at the instant. This is known as statistical law of radioactivity or Radioactive Decay Law.
Let `'N'` be the number of atoms of a radioactive sample at any instant. If `'dN'` is the number of, atoms which get disintegrated
in a small time `'dt'`
Rate of disintegration `= - (dN)/(dt)`
Negative sign indicates that the number of atoms decreases with increasing `'t'` .
According to the law of radioactive disintegration,
`-(dN)/(dt) prop N`
`(dN)/(dt) = - lamdaN`
where '`lamda`' is known as 'radioactive decay constant' and depends upon the nature of the substance
`int_(N_0)^N (dN)/N = - int_0^t lambda dt`
`ln N/(N_0) = - lamdat`
`N = N_0 e^(-lamdat)`
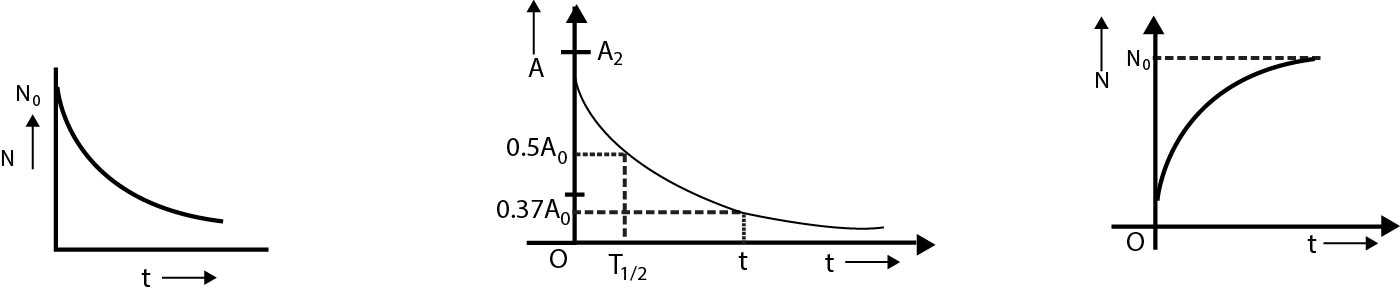
Variation of population of radioactive substances with time(fig=a)
we have `N = N_0 e^(-lamdat)` , here `lamda` is decay constant
`N_0=>` Initial no. of atoms (nuclei) in the sample
`N=>` No. of nuclei present in the sample at time `t` .
`N' =` no. decayed `= N_0 - N = N_0(1 - e^(- lamdat))`
Definition of decay constant `lamda`
at `t = 1/ lambda` , `lamdat = 1`
`N = N_0 e^(-lamdat) = N_0 e^(-1)`
`N = (N_0)/e`
`lamda=` reciprocal of time in which the number of atoms (nuclei) gets reduced to `1/e` times `N_0`.
`text(Half Life)``(t_(1/2))`
Time during which no. of nuclei reduces to half its original value.
We've got to find the time t after which `N = (N_0)/2`
we know `N = N_0 e^(- lamdat)`
`(N_0)/2 = N_0 e^(- lamdat)` , `e^( lamdat) = 2`
`lamdat = ln 2` , `t= (ln 2)/lambda = (0.693)/lambda`
`t_(t/2) = (0.693)/lambda`
`text(Average Life(Mean life))`
The nuclei of a radioactive substance are continuously disintegrating. This shows that life of every nucleus is different. It is impossible to tell when a particular nucleus will disintegrate because it all depends upon chance or probability. Moreover, the nucleus which disintegrates earlier has a shorter life while the nucleus which disintegrates later has a longer life. Therefore it is important to determine the mean or average life time of the nuclei.
`t_(av ) = (int_(N_0)^0 t dN)/(int_(N_0)^0 dN) = (int_(N_0)^0 tdN)/(-N_0)`
`(int_(N_0)^0 t lambda N_0 e^-(lamdat)dt)/(N_0) = int_0^(oo) lambda t e^(- lamdat) dt = 1/ lambda`
`text(Relation between Half Life, Average Life and Decay Constant)`
`t_(1/2) = (0.693)/lamda= 0.693t_(av)`
`t_(av) > t_(1/2)`
`text(Activity of Radioactive substance)` {fig-b}
The activity of a radioactive substance is defined as the rate of decay or the number of disintegration per second.
`A = -(dN)/(dt) = lamdaN = lamdaN_0 e^(- lamdat)`
`A = A_0 e^(- lamdat)`
where `A_0` is initial activity i.e., `A_0 = lamdaN_0`
The corresponding graph of `A` versus `t` is as shown in figure.
S. l. unit of activity is Becquerel (Bq)
`1` `Bq` `= 1` `dps` (disintegration per second)
`text(Remarks :)`
(i) Radioactivity of a substance is measured in following two units
(a) Curie : `text(1 curie)` `(Ci)` `= 3.7 xx 10^10` `text(disintegrations/sec)`
(b) Rutherford(rd) : `text(1 Rutherford)` `(rd)` `= 10^6` `text(disintegrations/sec)`
`text(1 Ci)` `= 3.7 xx 10^4quadrd`
(c) Sometimes in numerical problems specific activity is described which is defined as activity of radioactive substance per unit mass.
(ii) In numerical problems, it is convenient to use following relation
`N = N_0 (1/2)^n` {fig-c}
`n = ` Number of half lives `= t/(T_(1/2))`
(iii) Number of nuclei decayed up to to time `t` is given by
`N' = N_0 -N = N_0(1-e^(-lamdat))`
The variation of number of nuclei decayed with time 't' is as shown in figure-c.
(iv) Probability of a nucleus for survival up to time `'t'` is given by
`P = N/(N_0) = e^(- lamdat)`
(v) Probability of a nucleus to disintegrate in time `'t'` is given by
`P' = 1- e^(- lamdat)`
(vi) One of the situations of interest is, when radioactive nuclei are being produced at some constant rate a by nuclear reactions in an accelerator or a nuclear reactor.
Rate of accumulation `= (dN)/(dt) = a- lamdaN`
(Remember in this case also the activity is still the rate of decay which is `lamdaN`
Using initial condition that `N = N_0` at `t = 0` , the solution of this `text(differential equation)` is given by
`N = alpha/lambda (1- e^(- lamdat)) + N_0 e^(-lamdat)`
Let `'N'` be the number of atoms of a radioactive sample at any instant. If `'dN'` is the number of, atoms which get disintegrated
in a small time `'dt'`
Rate of disintegration `= - (dN)/(dt)`
Negative sign indicates that the number of atoms decreases with increasing `'t'` .
According to the law of radioactive disintegration,
`-(dN)/(dt) prop N`
`(dN)/(dt) = - lamdaN`
where '`lamda`' is known as 'radioactive decay constant' and depends upon the nature of the substance
`int_(N_0)^N (dN)/N = - int_0^t lambda dt`
`ln N/(N_0) = - lamdat`
`N = N_0 e^(-lamdat)`
Variation of population of radioactive substances with time(fig=a)
we have `N = N_0 e^(-lamdat)` , here `lamda` is decay constant
`N_0=>` Initial no. of atoms (nuclei) in the sample
`N=>` No. of nuclei present in the sample at time `t` .
`N' =` no. decayed `= N_0 - N = N_0(1 - e^(- lamdat))`
Definition of decay constant `lamda`
at `t = 1/ lambda` , `lamdat = 1`
`N = N_0 e^(-lamdat) = N_0 e^(-1)`
`N = (N_0)/e`
`lamda=` reciprocal of time in which the number of atoms (nuclei) gets reduced to `1/e` times `N_0`.
`text(Half Life)``(t_(1/2))`
Time during which no. of nuclei reduces to half its original value.
We've got to find the time t after which `N = (N_0)/2`
we know `N = N_0 e^(- lamdat)`
`(N_0)/2 = N_0 e^(- lamdat)` , `e^( lamdat) = 2`
`lamdat = ln 2` , `t= (ln 2)/lambda = (0.693)/lambda`
`t_(t/2) = (0.693)/lambda`
`text(Average Life(Mean life))`
The nuclei of a radioactive substance are continuously disintegrating. This shows that life of every nucleus is different. It is impossible to tell when a particular nucleus will disintegrate because it all depends upon chance or probability. Moreover, the nucleus which disintegrates earlier has a shorter life while the nucleus which disintegrates later has a longer life. Therefore it is important to determine the mean or average life time of the nuclei.
`t_(av ) = (int_(N_0)^0 t dN)/(int_(N_0)^0 dN) = (int_(N_0)^0 tdN)/(-N_0)`
`(int_(N_0)^0 t lambda N_0 e^-(lamdat)dt)/(N_0) = int_0^(oo) lambda t e^(- lamdat) dt = 1/ lambda`
`text(Relation between Half Life, Average Life and Decay Constant)`
`t_(1/2) = (0.693)/lamda= 0.693t_(av)`
`t_(av) > t_(1/2)`
`text(Activity of Radioactive substance)` {fig-b}
The activity of a radioactive substance is defined as the rate of decay or the number of disintegration per second.
`A = -(dN)/(dt) = lamdaN = lamdaN_0 e^(- lamdat)`
`A = A_0 e^(- lamdat)`
where `A_0` is initial activity i.e., `A_0 = lamdaN_0`
The corresponding graph of `A` versus `t` is as shown in figure.
S. l. unit of activity is Becquerel (Bq)
`1` `Bq` `= 1` `dps` (disintegration per second)
`text(Remarks :)`
(i) Radioactivity of a substance is measured in following two units
(a) Curie : `text(1 curie)` `(Ci)` `= 3.7 xx 10^10` `text(disintegrations/sec)`
(b) Rutherford(rd) : `text(1 Rutherford)` `(rd)` `= 10^6` `text(disintegrations/sec)`
`text(1 Ci)` `= 3.7 xx 10^4quadrd`
(c) Sometimes in numerical problems specific activity is described which is defined as activity of radioactive substance per unit mass.
(ii) In numerical problems, it is convenient to use following relation
`N = N_0 (1/2)^n` {fig-c}
`n = ` Number of half lives `= t/(T_(1/2))`
(iii) Number of nuclei decayed up to to time `t` is given by
`N' = N_0 -N = N_0(1-e^(-lamdat))`
The variation of number of nuclei decayed with time 't' is as shown in figure-c.
(iv) Probability of a nucleus for survival up to time `'t'` is given by
`P = N/(N_0) = e^(- lamdat)`
(v) Probability of a nucleus to disintegrate in time `'t'` is given by
`P' = 1- e^(- lamdat)`
(vi) One of the situations of interest is, when radioactive nuclei are being produced at some constant rate a by nuclear reactions in an accelerator or a nuclear reactor.
Rate of accumulation `= (dN)/(dt) = a- lamdaN`
(Remember in this case also the activity is still the rate of decay which is `lamdaN`
Using initial condition that `N = N_0` at `t = 0` , the solution of this `text(differential equation)` is given by
`N = alpha/lambda (1- e^(- lamdat)) + N_0 e^(-lamdat)`