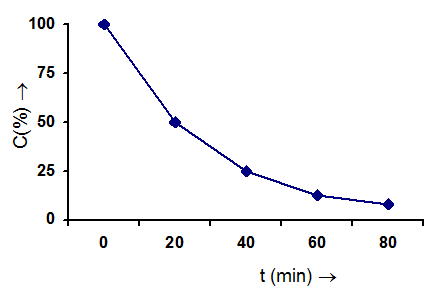
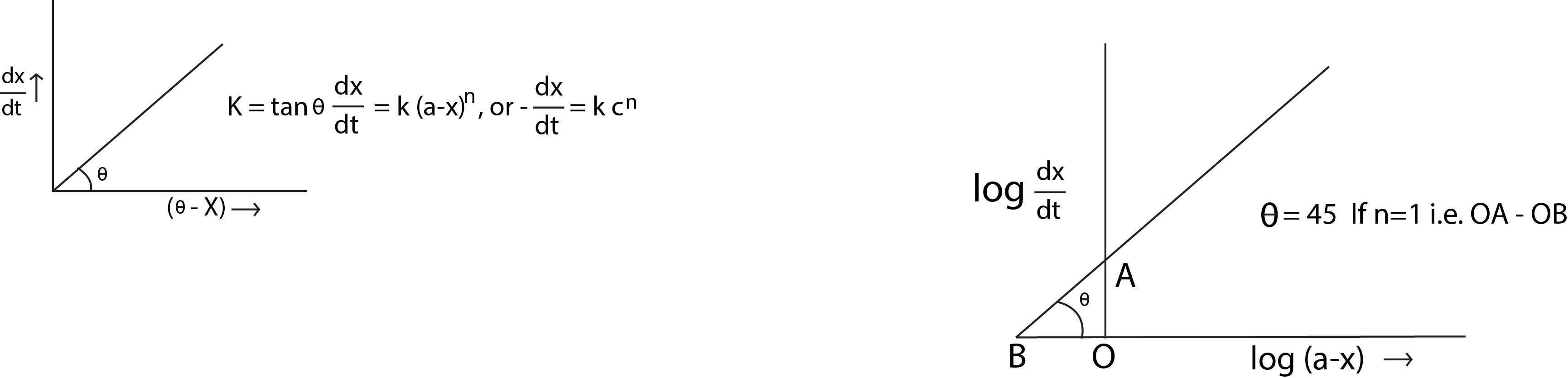Second Order Reaction :
`text(Type I)` :
`A->` Product
Initial concentration `a` `0` Concentration after time `t` `(a-x)` `x`
Differential rate law:
Integrated rate law (on integration of above equation):
`(d(a-x))/(dt)=+(dx)/(dt)=k_2(a-x)^2`
Integrated rate law (on integration of above equation):
`k_2=1/t x/(a(a-x))`
`text(Type II)` :
(i) Reactants `A` and `B` have the same initial concentration
`A + B ->` Products
Initial concentration `a` `0`
Concentration after time, `t` `(a-x)` `(a-x)` `x`
Differential Rate Law :
`-(d(a-x))/(dt)=+(dx)/(dt)-k_2(a-x)(a-x)-k_2(a-x)^2`
`k_2=1/t * x/(a(a-x))` (Same as for `A->` products)
(ii) Reactants `A` and `B` have different initial concentrations.
`A + B ->` products
Initial concentration `a` `b` `0`
Concentration after time, `t` `(a -x)` `(b-x)` `x`
Differential Rate Law:
`-(d(a-x))/(dt)=-(d(b-x))/(dt)=+(dx)/(dt)=k_2(a-x)(b-x)`
Integrated Rate Law :
When `a>b` : `k_2 = 2.303/(t(a-b)) log (b(a-x))/(a(b-x))`
when `b> a` : `k_2=2.303/(t(a-b)) log (a(b-x))/(b(a-x))`
The unit of the rate constant, `k_2` is `text(concentration) ^(-1) text(time)^(-1)`.
`A->` Product
Initial concentration `a` `0` Concentration after time `t` `(a-x)` `x`
Differential rate law:
Integrated rate law (on integration of above equation):
`(d(a-x))/(dt)=+(dx)/(dt)=k_2(a-x)^2`
Integrated rate law (on integration of above equation):
`k_2=1/t x/(a(a-x))`
`text(Type II)` :
(i) Reactants `A` and `B` have the same initial concentration
`A + B ->` Products
Initial concentration `a` `0`
Concentration after time, `t` `(a-x)` `(a-x)` `x`
Differential Rate Law :
`-(d(a-x))/(dt)=+(dx)/(dt)-k_2(a-x)(a-x)-k_2(a-x)^2`
`k_2=1/t * x/(a(a-x))` (Same as for `A->` products)
(ii) Reactants `A` and `B` have different initial concentrations.
`A + B ->` products
Initial concentration `a` `b` `0`
Concentration after time, `t` `(a -x)` `(b-x)` `x`
Differential Rate Law:
`-(d(a-x))/(dt)=-(d(b-x))/(dt)=+(dx)/(dt)=k_2(a-x)(b-x)`
Integrated Rate Law :
When `a>b` : `k_2 = 2.303/(t(a-b)) log (b(a-x))/(a(b-x))`
when `b> a` : `k_2=2.303/(t(a-b)) log (a(b-x))/(b(a-x))`
The unit of the rate constant, `k_2` is `text(concentration) ^(-1) text(time)^(-1)`.