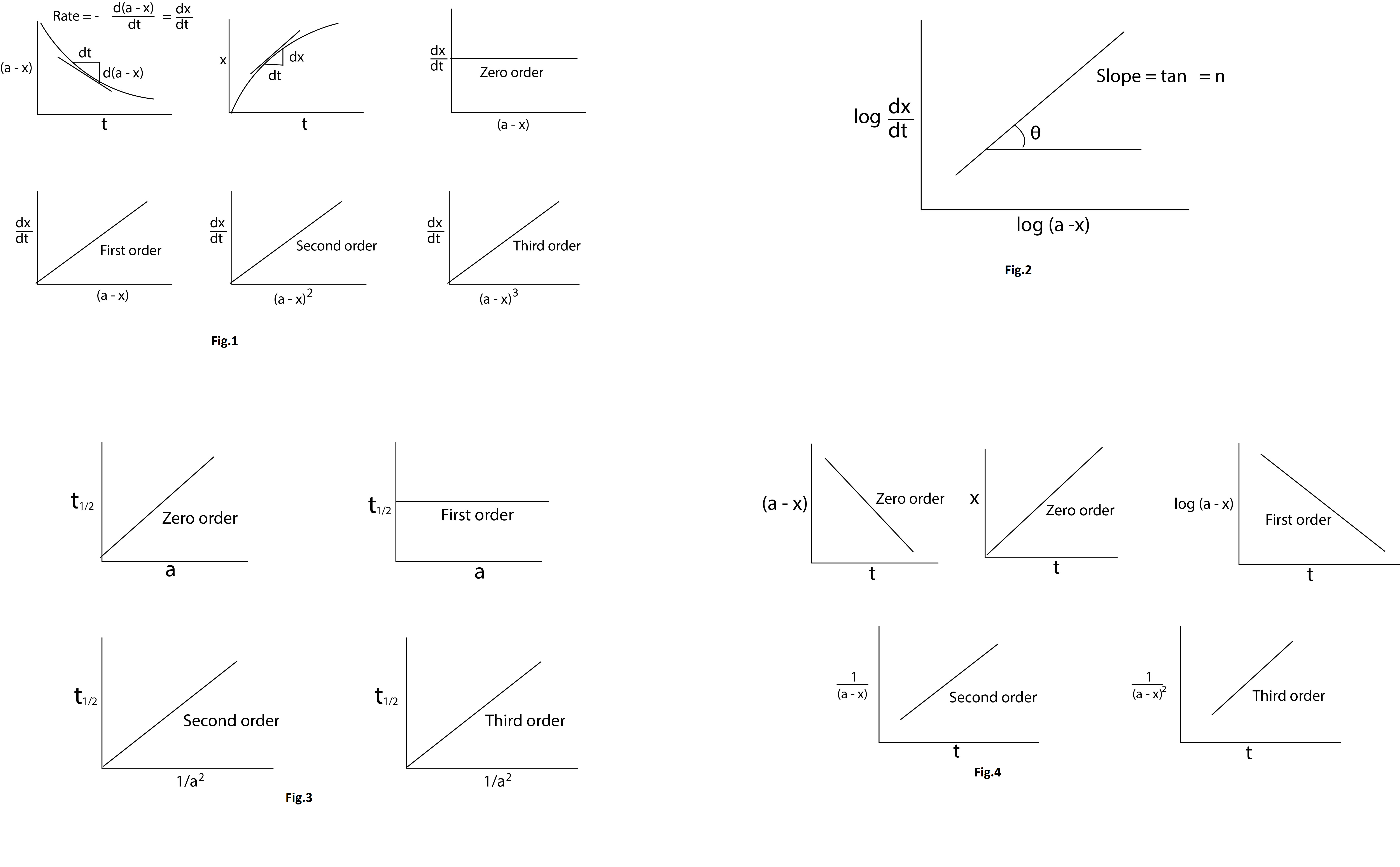
(a) A graph is plotted between time `t` and the concentration of reactant `(a-x)` or product `(x)`, slope of which gives the rate of reaction `(dx)/(dt)` for the selected time instant. (figure 1 & 2)The various values of the rate `((dx)/(dt))` are now plotted against the corresponding concentration `(a-x)` or `(a-x)^2` or `(a-x)^3` from which we draw the following graphical conclusions : See fig.1.
(b) Alternatively, the order (`n`) can also be determined from the slope of the curve plotted
between `log [(dx)/(dt)]` and `log (a-x)` : See fig.2.
`(dx)/(dt) = k(a-x)^n`
`log[(dx)/(dt)] = logk +nlog(a-x)`
(c)We know that the time required to complete a definite fraction (say one half) of the reaction depends on the initial concentration of the reactant in the following way :
For zero-order reaction, `t_(1//2) prop a`
i.e., `t_(1//2) prop 1/a^(-1)`
For first-order reaction, `t_(1//2)` is independent of `a`
i.e., `t_(1//2) prop 1/a^(0)`
For Second-order reaction, `t_(1//2) prop 1/a`
For third-order reaction, `t_(1//2) prop 1/a^2`
`:.` for `n^(th)`-order reaction, `t_(1//2) prop 1/a^(n-1)`
Thus we get the fo llowing plots for reactions of various orders : See fig.3.
(d) From integrated rate law equations of various reactions of different orders, we may have the following plots : See fig.4.
In case of gaseous reactions, concentration may be replaced by pressure.
(a) A graph is plotted between time `t` and the concentration of reactant `(a-x)` or product `(x)`, slope of which gives the rate of reaction `(dx)/(dt)` for the selected time instant. (figure 1 & 2)The various values of the rate `((dx)/(dt))` are now plotted against the corresponding concentration `(a-x)` or `(a-x)^2` or `(a-x)^3` from which we draw the following graphical conclusions : See fig.1.
(b) Alternatively, the order (`n`) can also be determined from the slope of the curve plotted
between `log [(dx)/(dt)]` and `log (a-x)` : See fig.2.
`(dx)/(dt) = k(a-x)^n`
`log[(dx)/(dt)] = logk +nlog(a-x)`
(c)We know that the time required to complete a definite fraction (say one half) of the reaction depends on the initial concentration of the reactant in the following way :
For zero-order reaction, `t_(1//2) prop a`
i.e., `t_(1//2) prop 1/a^(-1)`
For first-order reaction, `t_(1//2)` is independent of `a`
i.e., `t_(1//2) prop 1/a^(0)`
For Second-order reaction, `t_(1//2) prop 1/a`
For third-order reaction, `t_(1//2) prop 1/a^2`
`:.` for `n^(th)`-order reaction, `t_(1//2) prop 1/a^(n-1)`
Thus we get the fo llowing plots for reactions of various orders : See fig.3.
(d) From integrated rate law equations of various reactions of different orders, we may have the following plots : See fig.4.
In case of gaseous reactions, concentration may be replaced by pressure.