Parallel Reactions :
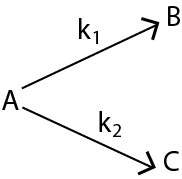
In such reactions (mostly organic) a single reactant gives two products `B` and `C` with different rate constants. If we assume that both
of them are first order, we get.
`-(d[A])/(dt)=k_1[A]+k_2[A]=k_1+k_2[A]`..........(1)
`(d[B])/(dt)=k_2[A]`..........(2)
And `(d[C])/(dt)=k_2[A]`.......(3)
Let us assume that in a time interval, `dt, x` moles/lit of `B` was produced and `y` moles/lit of `C` was produced.
`(d[B])/(dt)=x/(dt)` and `(d[C])/(dt)=v/(dt)` `((d[B])/(dt))/((d[C])/(dt))=x/y`.
We can also see that from (2) and (3), `((d[B])/(dt))/((d[C])/(dt)) =k_1/k_2`
`x/y=k_1/k_2` This means that irrespective of how much time is elapsed, the ratio of concentration of `B` to that of `C` from the start (assuming no `B` and `B` in the beginning) is a constant equal to `k_1//k_2`
of them are first order, we get.
`-(d[A])/(dt)=k_1[A]+k_2[A]=k_1+k_2[A]`..........(1)
`(d[B])/(dt)=k_2[A]`..........(2)
And `(d[C])/(dt)=k_2[A]`.......(3)
Let us assume that in a time interval, `dt, x` moles/lit of `B` was produced and `y` moles/lit of `C` was produced.
`(d[B])/(dt)=x/(dt)` and `(d[C])/(dt)=v/(dt)` `((d[B])/(dt))/((d[C])/(dt))=x/y`.
We can also see that from (2) and (3), `((d[B])/(dt))/((d[C])/(dt)) =k_1/k_2`
`x/y=k_1/k_2` This means that irrespective of how much time is elapsed, the ratio of concentration of `B` to that of `C` from the start (assuming no `B` and `B` in the beginning) is a constant equal to `k_1//k_2`