-Particle Scattering Experiment and Rutherford's Nuclear Model of Atom

At the suggestion of Ernst Rutherford, in 1911, H. Geiger and E. Marsden performed some experiments. In one of their experiments, as shown in Fig. a, they directed a beam of `5.5 MeV` `α`-particles emitted from a `text( )_(83)Bi^(214)` radioactive source at a thin metal foil made of gold. Figure-b shows a schematic diagram of this experiment. Alpha-particles emitted by a `text( )_(83)Bi^(214)` radioactive source were collimated into a narrow beam by their passage through lead bricks. The beam was allowed to fall on a thin foil of gold of thickness `2.1 xx 10^-7` `m`. The scattered alpha-particles were observed through a rotatable detector consisting of zinc sulphide screen and a microscope. The scattered alpha-particles on striking the screen produced brief light flashes or scintillations. These flashes may be
viewed through a microscope and the distribution of the number of scattered particles may be studied as a function of angle of scattering.
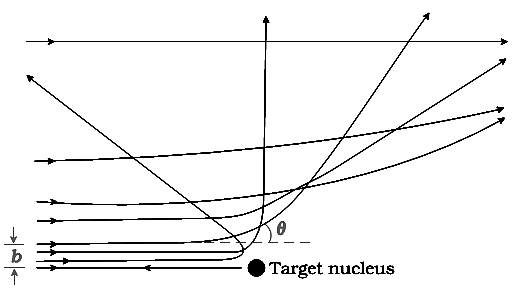
A typical graph of the total number of `α-` particles scattered at different angles, in a given interval of time, is shown in Fig-b . The dots in this figure represent the data points and the solid curve is the theoretical prediction based on the assumption that the target atom has a small, dense, positively charged nucleus. Many of the α-particles pass through the foil. It means that they do not suffer any collisions. Only about `0.14%` of the incident α-particles scatter by more than `1^@`, and about `1` in `8000` deflect by more than `90^@`. Rutherford argued that, to deflect the `α-` particle backwards, it must experience a large repulsive force. This force could be provided if the greater part of the mass of the atom and its positive charge were concentrated tightly at its centre. Then the incoming α-particle could get very close to the positive charge without penetrating it, and such a close encounter would result in a large deflection. This agreement supported the hypothesis of the nuclear atom. This is why Rutherford is credited with the discovery of the nucleus. In Rutherford's nuclear model of the atom, the entire positive charge and most of the mass of the atom are concentrated in the nucleus with the electrons some distance away. The electrons would be moving in orbits about the nucleus just as the planets do around the sun. Rutherford's experiments suggested the size of the nucleus to be about `10^(-15)` m to `10^(-14)` m. From kinetic theory, the size of an atom was known to be `10^(-10)` m, about 10,000 to 100,000 times larger than the size of the nucleus. Thus, the electrons would seem to be at a distance from the nucleus of about 10,000 to 100,000 times the size of the nucleus itself. Thus, most of an atom is empty space. With the atom being largely empty space, it is easy to see why most `α`-particles go right through a thin metal foil. However, when α-particle happens to come near a nucleus, the intense electric field there scatters it through a large angle. The atomic electrons, being so light, do not appreciably affect the `α`-particles. The scattering data shown in Fig-c can be analysed by employing Rutherford's nuclear model of the atom. As the gold foil is very thin, it can be assumed that `α`-particles will suffer not more than one scattering during their passage through it. Therefore, computation of the trajectory of an alpha-particle scattered by a single nucleus is enough. Alpha particles are nuclei of helium atoms and, therefore, carry two units, 2e, of positive charge and have the mass of the helium atom. The charge of the gold nucleus is `Ze`, where `Z` is the atomic number of the atom; for gold `Z = 79`. Since the nucleus of gold is about `50` times heavier than an α-particle, it is reasonable to assume that it remains stationary throughout the scattering process. Under these assumptions, the
The magnitude of this force is
`F = 1/(4 pi epsilon_0) xx ((2e)(Ze))/r^2.......................(1)`
where `r` is the distance between the `α`-particle and the nucleus. The force is directed along the line joining the `α`-particle and the nucleus. The magnitude and direction of the force on an `α`-particle continuously changes as it approaches the nucleus and recedes away from it.
viewed through a microscope and the distribution of the number of scattered particles may be studied as a function of angle of scattering.
A typical graph of the total number of `α-` particles scattered at different angles, in a given interval of time, is shown in Fig-b . The dots in this figure represent the data points and the solid curve is the theoretical prediction based on the assumption that the target atom has a small, dense, positively charged nucleus. Many of the α-particles pass through the foil. It means that they do not suffer any collisions. Only about `0.14%` of the incident α-particles scatter by more than `1^@`, and about `1` in `8000` deflect by more than `90^@`. Rutherford argued that, to deflect the `α-` particle backwards, it must experience a large repulsive force. This force could be provided if the greater part of the mass of the atom and its positive charge were concentrated tightly at its centre. Then the incoming α-particle could get very close to the positive charge without penetrating it, and such a close encounter would result in a large deflection. This agreement supported the hypothesis of the nuclear atom. This is why Rutherford is credited with the discovery of the nucleus. In Rutherford's nuclear model of the atom, the entire positive charge and most of the mass of the atom are concentrated in the nucleus with the electrons some distance away. The electrons would be moving in orbits about the nucleus just as the planets do around the sun. Rutherford's experiments suggested the size of the nucleus to be about `10^(-15)` m to `10^(-14)` m. From kinetic theory, the size of an atom was known to be `10^(-10)` m, about 10,000 to 100,000 times larger than the size of the nucleus. Thus, the electrons would seem to be at a distance from the nucleus of about 10,000 to 100,000 times the size of the nucleus itself. Thus, most of an atom is empty space. With the atom being largely empty space, it is easy to see why most `α`-particles go right through a thin metal foil. However, when α-particle happens to come near a nucleus, the intense electric field there scatters it through a large angle. The atomic electrons, being so light, do not appreciably affect the `α`-particles. The scattering data shown in Fig-c can be analysed by employing Rutherford's nuclear model of the atom. As the gold foil is very thin, it can be assumed that `α`-particles will suffer not more than one scattering during their passage through it. Therefore, computation of the trajectory of an alpha-particle scattered by a single nucleus is enough. Alpha particles are nuclei of helium atoms and, therefore, carry two units, 2e, of positive charge and have the mass of the helium atom. The charge of the gold nucleus is `Ze`, where `Z` is the atomic number of the atom; for gold `Z = 79`. Since the nucleus of gold is about `50` times heavier than an α-particle, it is reasonable to assume that it remains stationary throughout the scattering process. Under these assumptions, the
The magnitude of this force is
`F = 1/(4 pi epsilon_0) xx ((2e)(Ze))/r^2.......................(1)`
where `r` is the distance between the `α`-particle and the nucleus. The force is directed along the line joining the `α`-particle and the nucleus. The magnitude and direction of the force on an `α`-particle continuously changes as it approaches the nucleus and recedes away from it.