Energy Levels
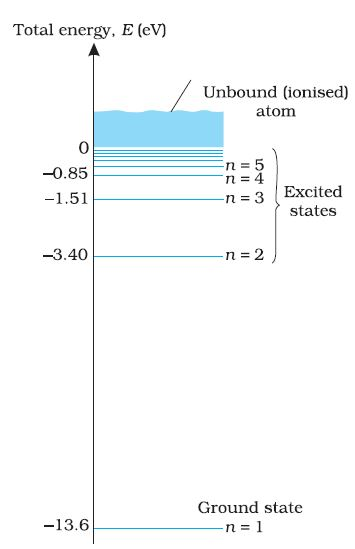
The energy of an atom is the least (largest negative value) when its electron is revolving in an orbit closest to the nucleus i.e., the one for which `n = 1`. For `n = 2, 3, ...` the absolute value of the energy `E` is smaller, hence the energy is progressively larger in the outer orbits. The lowest state of the atom, called the ground state, is that of the lowest energy, with the electron revolving in the orbit of smallest radius, the Bohr radius, `a_0`. The energy of this state (`n = 1`), `E_1` is `-13.6 eV`. Therefore, the minimum energy required to free the electron from the ground state of the hydrogen atom is `13.6 eV`. It is called the ionisation energy of the hydrogen atom. This prediction of the Bohr's model is in excellent agreement with the experimental value of ionisation energy.
The total energy of the electron in the stationary states of the hydrogen atom can be obtained by
`E_n= -(me^4)/(8n^2epsilon_0^2h^2)`
`E_n=-(13.6)/n^2 eV`..........(1)
At room temperature, most of the hydrogen atoms are in ground state. When a hydrogen atom receives energy by processes such as electron collisions, the atom may acquire sufficient energy to raise the electron to higher energy states. The atom is then said to be in an excited state. From Eq. (1), for `n = 2`; the energy `E_2` is `-3.40 eV`. It means that the energy required to excite an electron in hydrogen atom to its first excited state, is an energy equal to
`E_2 - E_1 = -3.40 eV - (-13.6) eV = 10.2 eV`
Similarly, `E_3 = -1.51 eV` and `E_3 - E_1 = 12.09 eV`,
or to excite the hydrogen atom from its ground state (`n = 1`) to second excited state (`n = 3`), `12.09 eV` energy is required, and so on. From these excited states the electron can then fall back to a state of lower energy, emitting a photon in the process. Thus, as the excitation of hydrogen atom increases (that is as `n` increases) the value of minimum energy required to free the electron from the excited atom decreases. The energy level diagram for the stationary states of a hydrogen atom, computed from Eq. (1), is given in Fig. The principal quantum number n labels the stationary states in the ascending order of energy. In this diagram, the highest energy state corresponds to `n =∞` in Eq, (1) and has an energy of `0 eV`. This is the energy of the atom when the electron is completely removed (`r = ∞`) from the nucleus and is at rest.
The total energy of the electron in the stationary states of the hydrogen atom can be obtained by
`E_n= -(me^4)/(8n^2epsilon_0^2h^2)`
`E_n=-(13.6)/n^2 eV`..........(1)
At room temperature, most of the hydrogen atoms are in ground state. When a hydrogen atom receives energy by processes such as electron collisions, the atom may acquire sufficient energy to raise the electron to higher energy states. The atom is then said to be in an excited state. From Eq. (1), for `n = 2`; the energy `E_2` is `-3.40 eV`. It means that the energy required to excite an electron in hydrogen atom to its first excited state, is an energy equal to
`E_2 - E_1 = -3.40 eV - (-13.6) eV = 10.2 eV`
Similarly, `E_3 = -1.51 eV` and `E_3 - E_1 = 12.09 eV`,
or to excite the hydrogen atom from its ground state (`n = 1`) to second excited state (`n = 3`), `12.09 eV` energy is required, and so on. From these excited states the electron can then fall back to a state of lower energy, emitting a photon in the process. Thus, as the excitation of hydrogen atom increases (that is as `n` increases) the value of minimum energy required to free the electron from the excited atom decreases. The energy level diagram for the stationary states of a hydrogen atom, computed from Eq. (1), is given in Fig. The principal quantum number n labels the stationary states in the ascending order of energy. In this diagram, the highest energy state corresponds to `n =∞` in Eq, (1) and has an energy of `0 eV`. This is the energy of the atom when the electron is completely removed (`r = ∞`) from the nucleus and is at rest.