Chemical Effect of current
The flow of electric current in a circuit is affected by a number of factors. We shall discuss in detail these effects but first let us have a glimpse of the terms used in this context:
`text(Chemical Energy)`
The energy stored in bonds of chemical compounds is called the chemical reaction. This energy is generally released in a chemical reaction which also produces heat as a by-product. When the chemical energy is released from a substance, it gets transformed into an entirely new substance. Some examples of stored chemical energy include battery, biomass, petroleum, natural gas, coal etc.
`text(Electrical Energy)`
The energy carried and transmitted by moving electrons in an electric conductor is called the electrical energy. This form of energy can be used and transmitted very easily but cannot be seen. The electricity generating plants do not create energy. They just transform the other form of energy into electricity. A battery transforms stored chemical energy in the form of electrons moving through the wire. Lightning is an apt example of electrical energy in nature.
`text(Electrolysis:)`
Behavior of substances to the passage of electric current through them can be classified into two categories:
(a) Substances which do not decompose due to the passage of electric current.
Example: Pure metals whether they are in solid or liquid form
(b) Substances which decompose due to the passage of electricity.
Example: Solution of copper sulphate, sodium chloride, etc. Such substances are called electrolytes.
The process of decomposition of electrolytes by the passage of electric current through them is called electrolysis.
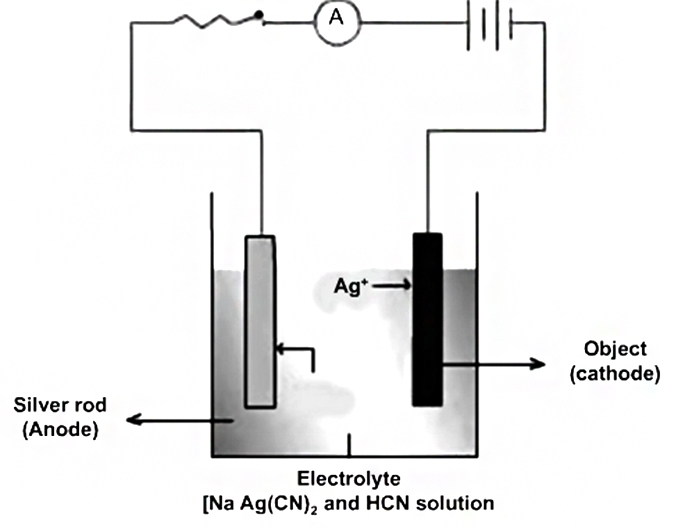
The process of electrolysis is carried in a vessel called voltmeter. The electrolyte is put into the voltmeter. A source of e.m.f is connected across two conducting plates dipping into electrolyte.
The plates are called electrodes. The plate A connected with positive terminal of the source is called anode whereas the plate C connected with negative terminal is called cathode.
`text(Faraday-s laws of electrolysis)`
Faraday gave the following two laws:
(a) The mass of ion deposited of an electrode in the process of electrolysis, is proportional to the quantity of charge that has passed through the electrolyte.
If -m' is the mass of ion deposited on an electrode due to passage of charge -q-, then,
m ∝ q
But q = it
Here, -i- is the current which flows for a time t.
So, m ∝ it
Or, m = zit -.... (1)
Here -z- is the constant of proportionality and is known as electro-chemical equivalent (e.c.e.) of the substance.
If i = 1 A, t = 1 s, m = z.
Therefore, electro- chemical equivalent of a substance is numerically equal to the mass of ion deposited when a charge of 1 C (or a current of 1 A passes through it for 1 s) passes through it.
e.c.e. varies from one substance to the other. Its units are `gC^-1.`
(b) When same current passes through several electrolytes for the same time, the masses of various ions deposited at each of the electrodes are proportional to their chemical equivalents (equivalent weights).
If certain current passes through two electrolytes A and B for the same time,
weight of A deposited / weight of B deposited = chemical equivalent of A / chemical equivalent of B
It may be noted that chemical equivalent of an element is defined as the ratio of its atomic weight to its valence.
Chemical equivalent = atomic weight/valence
Let same charge -q- be passed through two electrolytes having e.c.e.-s z1 and z2. Let m1 and m2 be the masses of ions liberated.
According to Faraday-s first law,
`m_1 = z_1q`
`m_2 = z_2q` or` m_1/m_2 = z_1/z_2 ` -... (2)
According to Faraday-s second law, `m_1/m_2 = E_1/E_2` -... (3)
Here, -E1- and -E2- are the chemical equivalents of two electrolytes.
From equations (2) and (3), we get,
`E_1/E_2 = z_1/z_2 or E_1/z_1 = E_2/z_2`
That is, `E/z` = constant = F (say) -... (4)
-F- is known as a -faraday-.
from equation (1), `z = m/it = m/q`
Substituting in (4), we get,
`F = E / (m/q) = Eq/m`
If `m = E, F = q`
Thus, a faraday is the amount of charge required to liberate one gram equivalent of a substance during electrolysis.
For copper `E = 31.5 g`
`z = 0.000329 gC^-1`
Substituting in equation (4), we get,
`F = 31.5 g / 0.000329 gC^-1 = 96500 C`
Thus, a faraday represents a charge `96500 C.`
`text(Chemical Energy)`
The energy stored in bonds of chemical compounds is called the chemical reaction. This energy is generally released in a chemical reaction which also produces heat as a by-product. When the chemical energy is released from a substance, it gets transformed into an entirely new substance. Some examples of stored chemical energy include battery, biomass, petroleum, natural gas, coal etc.
`text(Electrical Energy)`
The energy carried and transmitted by moving electrons in an electric conductor is called the electrical energy. This form of energy can be used and transmitted very easily but cannot be seen. The electricity generating plants do not create energy. They just transform the other form of energy into electricity. A battery transforms stored chemical energy in the form of electrons moving through the wire. Lightning is an apt example of electrical energy in nature.
`text(Electrolysis:)`
Behavior of substances to the passage of electric current through them can be classified into two categories:
(a) Substances which do not decompose due to the passage of electric current.
Example: Pure metals whether they are in solid or liquid form
(b) Substances which decompose due to the passage of electricity.
Example: Solution of copper sulphate, sodium chloride, etc. Such substances are called electrolytes.
The process of decomposition of electrolytes by the passage of electric current through them is called electrolysis.
The process of electrolysis is carried in a vessel called voltmeter. The electrolyte is put into the voltmeter. A source of e.m.f is connected across two conducting plates dipping into electrolyte.
The plates are called electrodes. The plate A connected with positive terminal of the source is called anode whereas the plate C connected with negative terminal is called cathode.
`text(Faraday-s laws of electrolysis)`
Faraday gave the following two laws:
(a) The mass of ion deposited of an electrode in the process of electrolysis, is proportional to the quantity of charge that has passed through the electrolyte.
If -m' is the mass of ion deposited on an electrode due to passage of charge -q-, then,
m ∝ q
But q = it
Here, -i- is the current which flows for a time t.
So, m ∝ it
Or, m = zit -.... (1)
Here -z- is the constant of proportionality and is known as electro-chemical equivalent (e.c.e.) of the substance.
If i = 1 A, t = 1 s, m = z.
Therefore, electro- chemical equivalent of a substance is numerically equal to the mass of ion deposited when a charge of 1 C (or a current of 1 A passes through it for 1 s) passes through it.
e.c.e. varies from one substance to the other. Its units are `gC^-1.`
(b) When same current passes through several electrolytes for the same time, the masses of various ions deposited at each of the electrodes are proportional to their chemical equivalents (equivalent weights).
If certain current passes through two electrolytes A and B for the same time,
weight of A deposited / weight of B deposited = chemical equivalent of A / chemical equivalent of B
It may be noted that chemical equivalent of an element is defined as the ratio of its atomic weight to its valence.
Chemical equivalent = atomic weight/valence
Let same charge -q- be passed through two electrolytes having e.c.e.-s z1 and z2. Let m1 and m2 be the masses of ions liberated.
According to Faraday-s first law,
`m_1 = z_1q`
`m_2 = z_2q` or` m_1/m_2 = z_1/z_2 ` -... (2)
According to Faraday-s second law, `m_1/m_2 = E_1/E_2` -... (3)
Here, -E1- and -E2- are the chemical equivalents of two electrolytes.
From equations (2) and (3), we get,
`E_1/E_2 = z_1/z_2 or E_1/z_1 = E_2/z_2`
That is, `E/z` = constant = F (say) -... (4)
-F- is known as a -faraday-.
from equation (1), `z = m/it = m/q`
Substituting in (4), we get,
`F = E / (m/q) = Eq/m`
If `m = E, F = q`
Thus, a faraday is the amount of charge required to liberate one gram equivalent of a substance during electrolysis.
For copper `E = 31.5 g`
`z = 0.000329 gC^-1`
Substituting in equation (4), we get,
`F = 31.5 g / 0.000329 gC^-1 = 96500 C`
Thus, a faraday represents a charge `96500 C.`