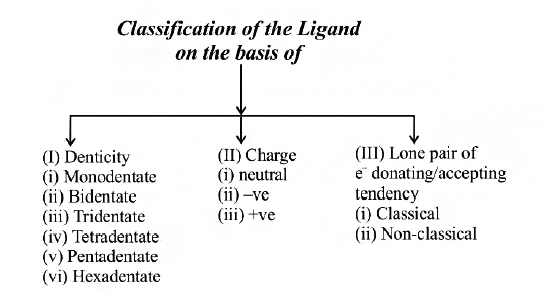
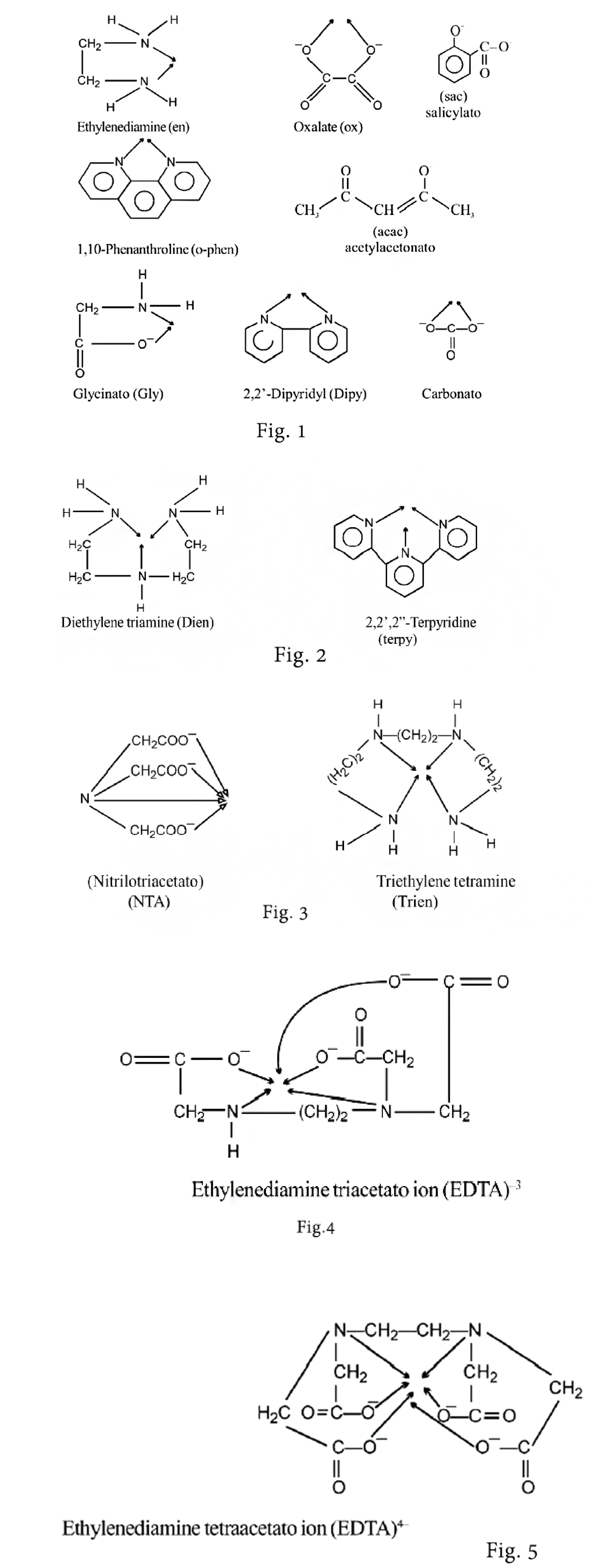
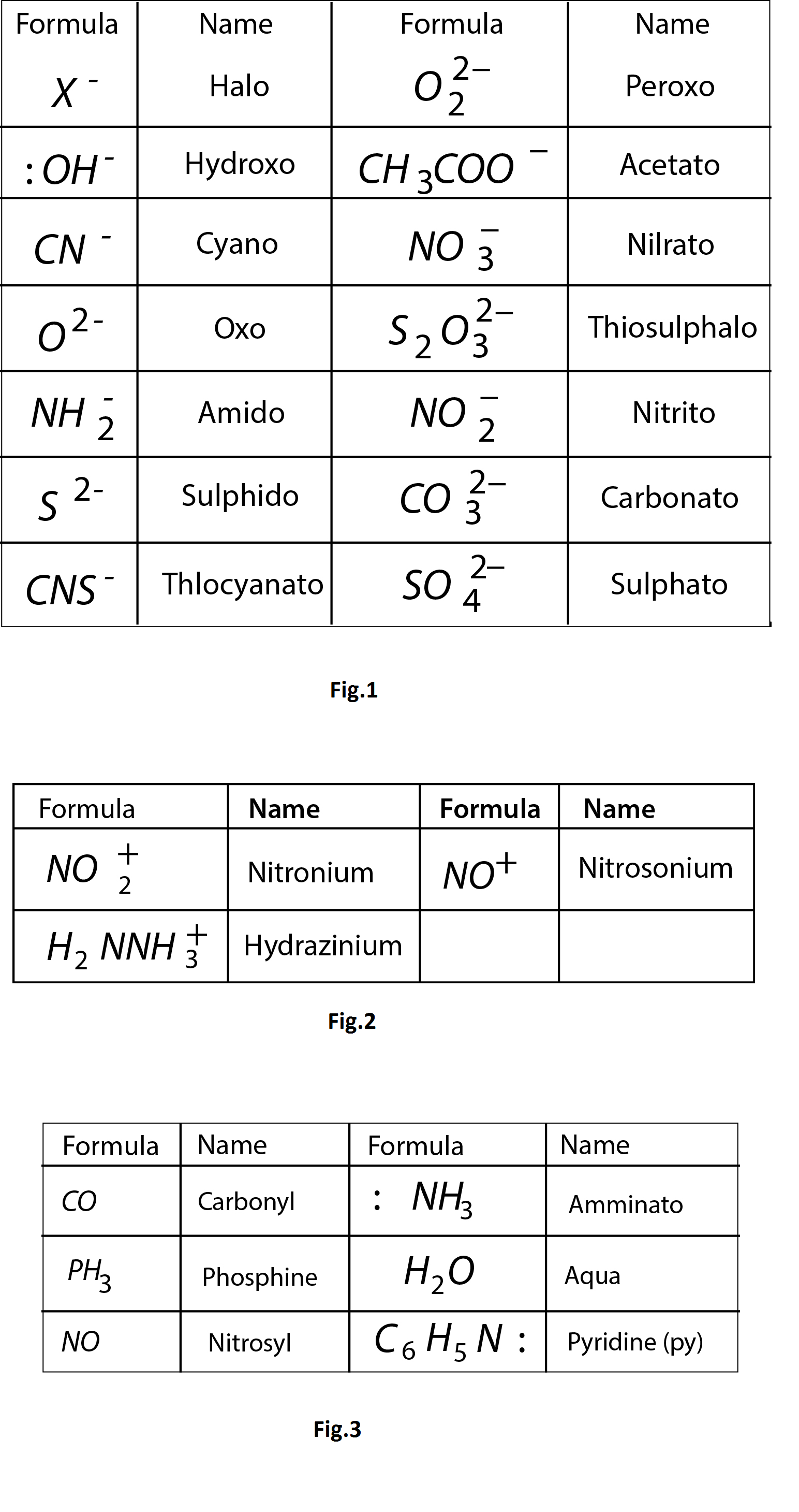
(A) Ligands having one (or more) lone pair (or pairs) of electrons
a) Ligands which contain vacant `pi`-type orbitals that can receive back donated `pi`-electron from metal ion in low oxidation state.
e.g.: `CO`, `NO`, `CN^-`, `NC^-` , `R-Nunderset(rightarrow)(=)C`, `R_3P`, `R_3As`, `alpha,alpha-text(dipyridyl)`, `o-text(phenanthroline)`
b) All these ligands also have filled donor orbital in addition to vacant `pi`-type acceptor orbitals.
c) Thus in these complexes both metal and ligand function as donors and acceptors
(`M overset(sigma) underset(pi)rightleftarrow L`)
d) Ligands which do not have vacant orbitals to receive back donated electron from metals. e.g. `H_2O`, `NH_3`, `F^-`.
(B) Ligands having no lone pairs of electrons but have `pi` bonding electron
e.g. Ethylene, benzene, cyclopentadienyl-ion, `K[PtCl_3(C_2H_4)] text(Zisses salt)`
(A) Ligands having one (or more) lone pair (or pairs) of electrons
a) Ligands which contain vacant `pi`-type orbitals that can receive back donated `pi`-electron from metal ion in low oxidation state.
e.g.: `CO`, `NO`, `CN^-`, `NC^-` , `R-Nunderset(rightarrow)(=)C`, `R_3P`, `R_3As`, `alpha,alpha-text(dipyridyl)`, `o-text(phenanthroline)`
b) All these ligands also have filled donor orbital in addition to vacant `pi`-type acceptor orbitals.
c) Thus in these complexes both metal and ligand function as donors and acceptors
(`M overset(sigma) underset(pi)rightleftarrow L`)
d) Ligands which do not have vacant orbitals to receive back donated electron from metals. e.g. `H_2O`, `NH_3`, `F^-`.
(B) Ligands having no lone pairs of electrons but have `pi` bonding electron
e.g. Ethylene, benzene, cyclopentadienyl-ion, `K[PtCl_3(C_2H_4)] text(Zisses salt)`