Isomerism in Co-ordination Compounds :
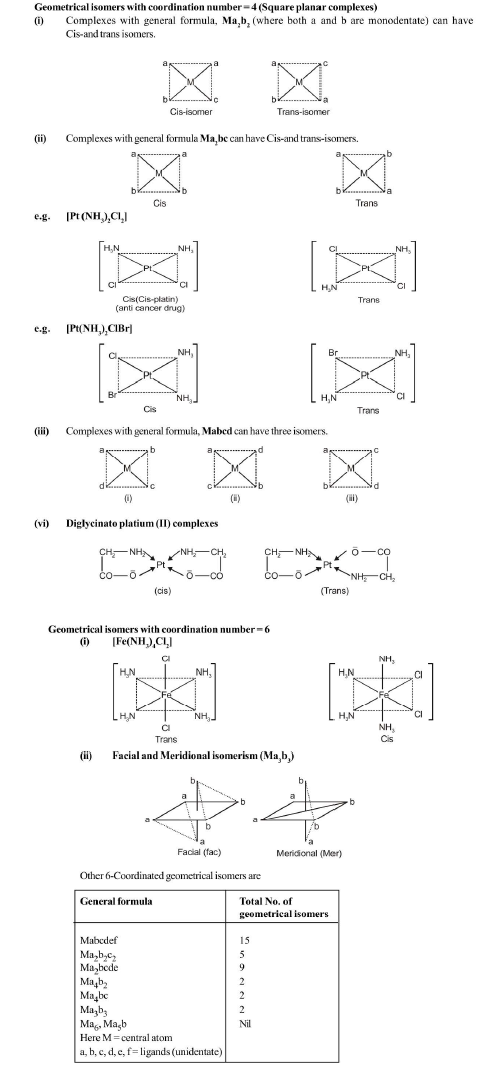
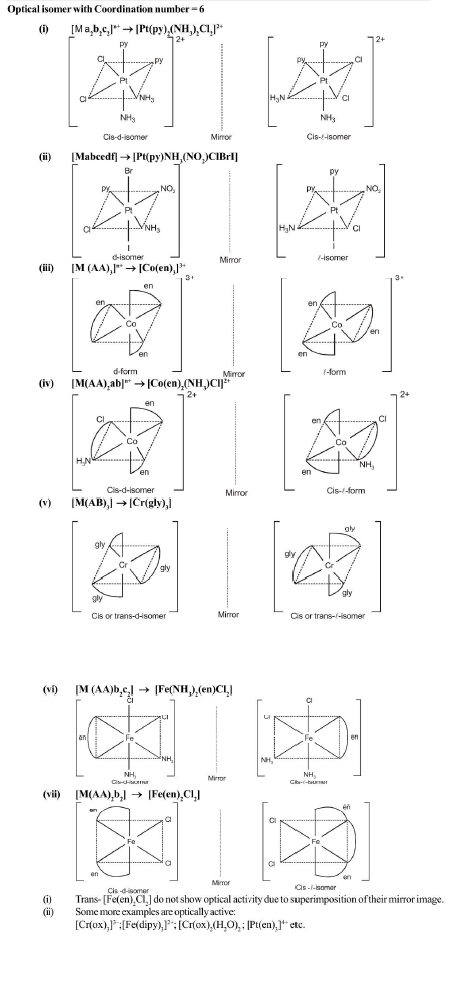
Compounds having the same molecular formula but different structures or spatial arrangements are called isomers and the phenomenon is referred as isomerism. Isomerism is commonly considered, to be the characteristic of only organic compounds, it is also found although less frequently among inorganic substances.
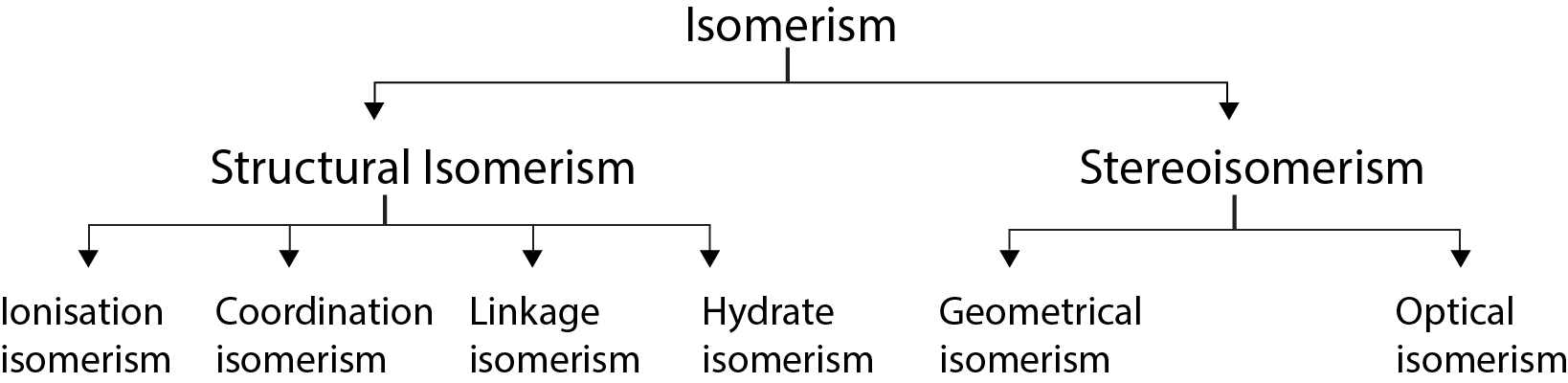
Classification of Isomerism : See fig.
Classification of Isomerism : See fig.