Transition Elements
They are often called 'transition elements' because
(i) They show's variable oxidation state in their compounds.
(ii) There outermost (`n^(th)` )and as well as penultimate (`n - 1`) shell is incomplete.
(iii) Their position in the periodic table is between `s`-block and `p`-block elements.
Typically, the transition elements have an incompletely filled `d`-level. Since `Zn` group has `d^10` configuration and are not considered as transition elements but they are `d`-block elements.
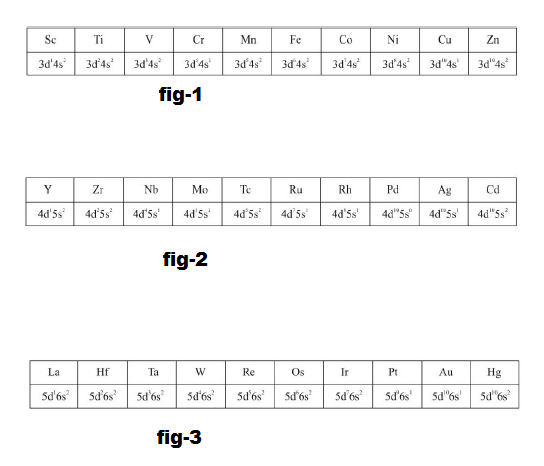
(i) `text(The first transition series)`: (`3d` series) involves the filling of `3d` orbitals and has `10` elements from scandium (`Z =21`) to zinc (`Z= 30`). See fig.1
(ii) `text(The second transition series)`: (`4d` series) involves the filling of `4d` orbitals and has `10` elements from ytterium (`Z=39`) to cadmium (`Z=48`). See fig.2
(iii) `text(The third transition series)`: (`5d` series) involves the filling of `5d` oroitals and has `10` elements. The first element of this series is lanthanum (`Z= 57`). It is followed by `14` elements (lanthanides, involving filling of `4f` orbitals). The next nine elements are from hafnium (`Z=72`) to mercury (`Z=80`). See fig.3
(iv) `text(The fourth transition series)` is incomplete and contains only three elements `text()_(89)Ac`, `text()_(104)Rf`, `text()_(105)Ha`.
`Zn` (`30`) is `[Ar]4s^2 3d^10`
`Cd` (`48`) is `[Kr]5s^2 4d^10`
`Hg` (`80`) is `[Xe]6s^2 4f^14 5d^10`
These elements have completely filled `(n-1 )d` subshell in their elementary as well as ionic state and so are not true transition metals. Their properties are quite different from those of transition metals. `Zn` is used in galvanizing, in making alloys, in making white pigment and in rubber industry (`ZnO` acts as filler). `Cd` is used in nuclear reactors as moderators, in making `Ni-Cd` storage cells and in making paints.
Mercury is used in scientific equipments and in electrolytic cells. Several compounds of `Hg` are used in making antiseptics.
(i) They show's variable oxidation state in their compounds.
(ii) There outermost (`n^(th)` )and as well as penultimate (`n - 1`) shell is incomplete.
(iii) Their position in the periodic table is between `s`-block and `p`-block elements.
Typically, the transition elements have an incompletely filled `d`-level. Since `Zn` group has `d^10` configuration and are not considered as transition elements but they are `d`-block elements.
(i) `text(The first transition series)`: (`3d` series) involves the filling of `3d` orbitals and has `10` elements from scandium (`Z =21`) to zinc (`Z= 30`). See fig.1
(ii) `text(The second transition series)`: (`4d` series) involves the filling of `4d` orbitals and has `10` elements from ytterium (`Z=39`) to cadmium (`Z=48`). See fig.2
(iii) `text(The third transition series)`: (`5d` series) involves the filling of `5d` oroitals and has `10` elements. The first element of this series is lanthanum (`Z= 57`). It is followed by `14` elements (lanthanides, involving filling of `4f` orbitals). The next nine elements are from hafnium (`Z=72`) to mercury (`Z=80`). See fig.3
(iv) `text(The fourth transition series)` is incomplete and contains only three elements `text()_(89)Ac`, `text()_(104)Rf`, `text()_(105)Ha`.
`Zn` (`30`) is `[Ar]4s^2 3d^10`
`Cd` (`48`) is `[Kr]5s^2 4d^10`
`Hg` (`80`) is `[Xe]6s^2 4f^14 5d^10`
These elements have completely filled `(n-1 )d` subshell in their elementary as well as ionic state and so are not true transition metals. Their properties are quite different from those of transition metals. `Zn` is used in galvanizing, in making alloys, in making white pigment and in rubber industry (`ZnO` acts as filler). `Cd` is used in nuclear reactors as moderators, in making `Ni-Cd` storage cells and in making paints.
Mercury is used in scientific equipments and in electrolytic cells. Several compounds of `Hg` are used in making antiseptics.