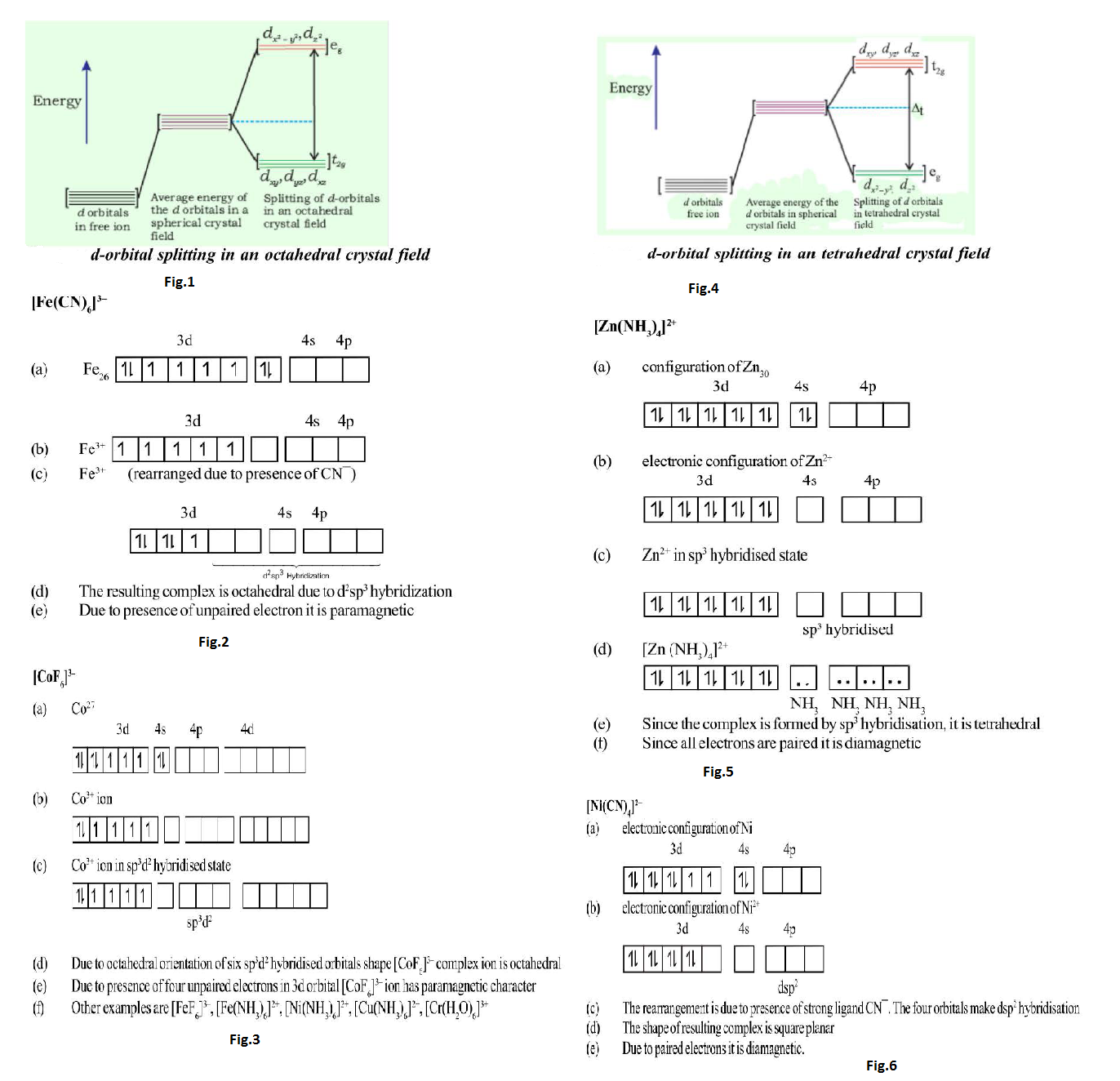
Valence Bond theory of Co-ordination Compounds :
(i) The suitable number of atomic orbitals of central metal ion (`s`, `p`, `d`) hybridise to provide empty hybrid orbitals.
(ii) These hybrid orbitals accept lone pair of electrons from the ligands and are directed towards the ligand positions according to the geometry of the complex.
(iii) When inner `d`-orbitals i.e. (`n-1`) `d` orbitals are used in hybridization, the complex is called-inner orbital or spin or hyperligated complex.
(iv) A substance which do not contain any unpaired electron is not attracted by `2` magnet. It is said to be diamagnetic. On the other hand, a substance which contains one or more unpaired electrons in the `d`-orbitals, is attracted by a magnetic field. [exception `O_2` and `NO`). It is said to be paramagnetic.
Paramagnetism can be calculated by the expression,
`mu_s` = `sqrt[n(n + 2)]`, where `mu` = magnetic moment.
`s` = spin only value and `n`= number of unpaired electrons.
On the basis of value of magnetic moment, we can predict the number of unpaired electrons present in the complex. If we know the number of unpaired electrons in the metal complex, then it is possible to predict the geometry of the complex species.
(v) There are two types of ligands namely strong field and weak field ligands. A strong field ligand is capable of forcing the electrons of the metal atom/ion to pair up (if required). Pairing is done only to the extent which is required to cause the hybridization possible for that Coordination number. A weak field ligand is incapable of making the electrons of the metal atom/ion to pair up.
Strong field ligands : `CN^-`, `CO`, `en`, `NH_3`, `H_2O`, `NO^-`, `Py`.
Weak field ligands : `I^-`, `Br^-`, `Cl^-`, `F^-`, `NO_3^-`, `OH^-`, `C_2O_4^(2-)`, `NH_3`, `H_2O`
Draw back of valence bond Theory :
(i) It describes bonding in coordination compounds only qualitatively.
(ii) It does not offer any explanation for the optical absorption spectra of complex.
(iii) It does not describe the detailed magnetic properties of coordination compounds.
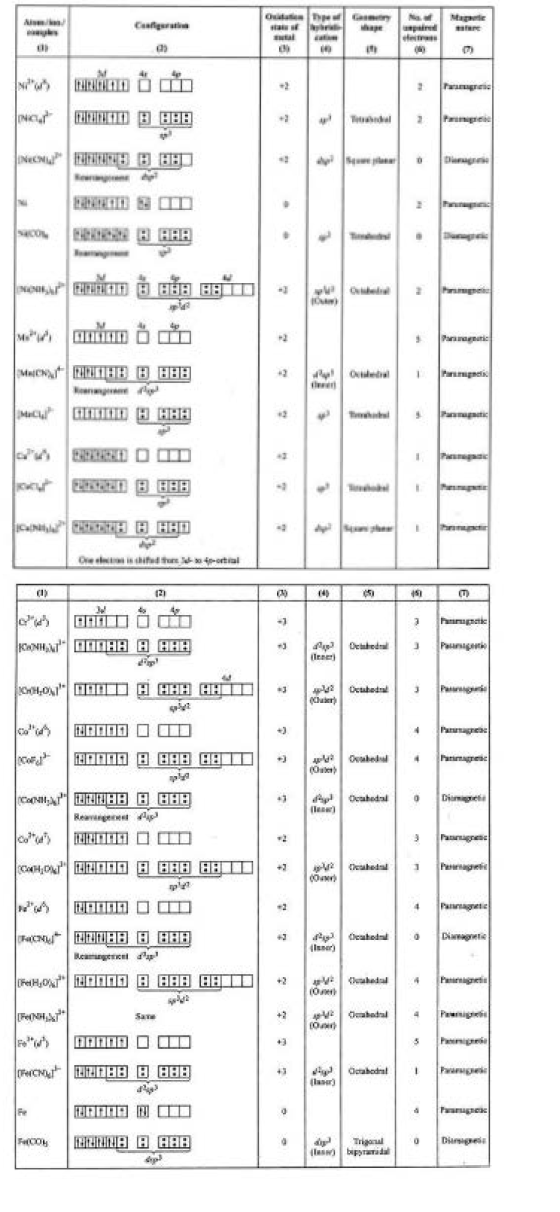
Geometry(shape) & magnetic nature of some the complexes (Application of valence bond theory) is given in fig.
(ii) These hybrid orbitals accept lone pair of electrons from the ligands and are directed towards the ligand positions according to the geometry of the complex.
(iii) When inner `d`-orbitals i.e. (`n-1`) `d` orbitals are used in hybridization, the complex is called-inner orbital or spin or hyperligated complex.
(iv) A substance which do not contain any unpaired electron is not attracted by `2` magnet. It is said to be diamagnetic. On the other hand, a substance which contains one or more unpaired electrons in the `d`-orbitals, is attracted by a magnetic field. [exception `O_2` and `NO`). It is said to be paramagnetic.
Paramagnetism can be calculated by the expression,
`mu_s` = `sqrt[n(n + 2)]`, where `mu` = magnetic moment.
`s` = spin only value and `n`= number of unpaired electrons.
On the basis of value of magnetic moment, we can predict the number of unpaired electrons present in the complex. If we know the number of unpaired electrons in the metal complex, then it is possible to predict the geometry of the complex species.
(v) There are two types of ligands namely strong field and weak field ligands. A strong field ligand is capable of forcing the electrons of the metal atom/ion to pair up (if required). Pairing is done only to the extent which is required to cause the hybridization possible for that Coordination number. A weak field ligand is incapable of making the electrons of the metal atom/ion to pair up.
Strong field ligands : `CN^-`, `CO`, `en`, `NH_3`, `H_2O`, `NO^-`, `Py`.
Weak field ligands : `I^-`, `Br^-`, `Cl^-`, `F^-`, `NO_3^-`, `OH^-`, `C_2O_4^(2-)`, `NH_3`, `H_2O`
Draw back of valence bond Theory :
(i) It describes bonding in coordination compounds only qualitatively.
(ii) It does not offer any explanation for the optical absorption spectra of complex.
(iii) It does not describe the detailed magnetic properties of coordination compounds.
Geometry(shape) & magnetic nature of some the complexes (Application of valence bond theory) is given in fig.