Effective Atomic Number (E.A.N.) Rule or Sidgwicks Rule :
According to sidgwick, metal atom present in coordination compound continues to accept electron pairs donated by the ligands till the total number of electrons on metal atom and those donated by ligands reaches to next noble gas configuration. This is known as Effective atomic number (E.A.N.) rule or sidgwick rule. It is calculated by the following formula
`text(E.A.N.) = text(Atomic number) - text(Oxidation number) + text(Coordination number) xx 2`
Example : Effective atomic number of cobalt in `[Co(NH_3)_6]^(3+)` can be calculated as follows :
Atomic number of cobalt = `27`
Oxidation state of cobalt in complex = `+3`
Number of electrons in `Co^(+3)` ion are (`27-3` =` 24`)
During coordinate covalent bonding, `Co^(+3)` ion gains `6` pairs of electrons.
Thus Effective atomic number of cobalt in `[Co(NH_3)_6]^(+3)` is `24 + 12 = 36`
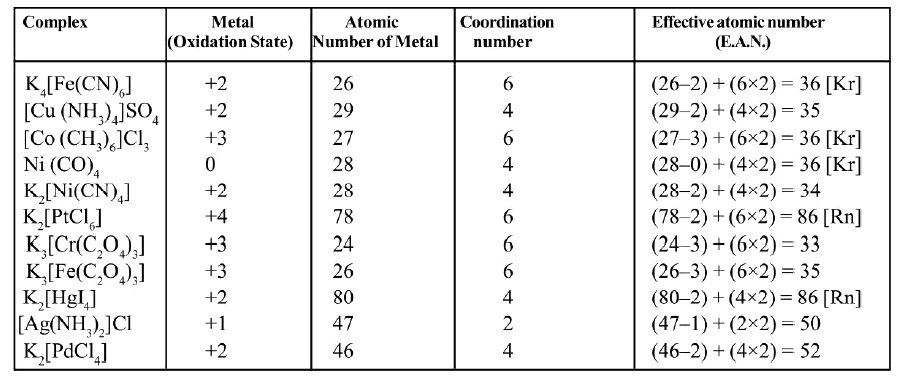
Some more examples are given in the table.
`text(E.A.N.) = text(Atomic number) - text(Oxidation number) + text(Coordination number) xx 2`
Example : Effective atomic number of cobalt in `[Co(NH_3)_6]^(3+)` can be calculated as follows :
Atomic number of cobalt = `27`
Oxidation state of cobalt in complex = `+3`
Number of electrons in `Co^(+3)` ion are (`27-3` =` 24`)
During coordinate covalent bonding, `Co^(+3)` ion gains `6` pairs of electrons.
Thus Effective atomic number of cobalt in `[Co(NH_3)_6]^(+3)` is `24 + 12 = 36`
Some more examples are given in the table.