COMPOUNDS OF SILVER
In the same way in presence of `O_2`, `Ag` complexes with `NaCN // KCN` .
`4Ag + 8KCN + 2H_2O + O_2 -> 4K[Ag(CN)_2] + 4KOH`
`AgNO_3`
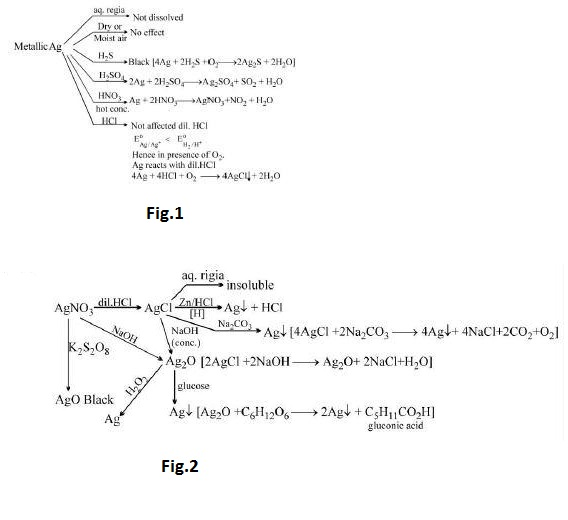
`text(Preparation)` : See fig.1.
`text(Properties)` : (i) It is called as lunar caustic because in contact with skin it produces burning sensation like that of caustic soda with the formation of finely devided silver (black colour).
(ii) Props. of `AgNO_3` :
`undersettext[(excess)][6AgNO_3] + 3I_2 + 3H_2O -> 5AgI + AgIO_3 + 6HNO_3`
(iii) `Ag_2SO_4 overset(Delta)-> 2Ag + SO_2 + O_2`
(iv) `Ag_2S_2O_3 + H_2O oversettext(Delta) -> Ag_2S + H_2SO_4`
`AgCl. AgBr. AgI` (but not `Ag_2S` ) are soluble in `Na_2S_2O_3` forming `[Ag(S_2O_3)_2]^(-3)` complexes
`AgBr : AgNO_3 overset(KBr) -> AgBr downarrow + KNO_3`
`text(Heating effect)` :
`2AgNO_3 overset(212^oC)-> 2AgNO_2 + O_2`
`2AgNO_2 overset(500^oC) -> 2Ag + 2NO + O_2`
`text(Other reactions)` : See fig.2.
`Ag_2O + H_2O_2 -> 2Ag + H_2O + O_2`
`K_2S_2O_8 + 2AgNO_3 + 2H_2O -> 2AgO + 2KHSO_4 + 2HNO_3`
`AgO` supposed to be paramagnetic due to `d^9` configuration. But actually it is diamagnetic and exists as `Ag^I[Ag^(III)O_2 ]`
`text(Reaction involved in developer)` :
`K_2Fe^(II)(C_2O_4)_2 + AgBr -> KFe^(III) (C_2O_4)_2 + Ag downarrow + KBr`
`4Ag + 8KCN + 2H_2O + O_2 -> 4K[Ag(CN)_2] + 4KOH`
`AgNO_3`
`text(Preparation)` : See fig.1.
`text(Properties)` : (i) It is called as lunar caustic because in contact with skin it produces burning sensation like that of caustic soda with the formation of finely devided silver (black colour).
(ii) Props. of `AgNO_3` :
`undersettext[(excess)][6AgNO_3] + 3I_2 + 3H_2O -> 5AgI + AgIO_3 + 6HNO_3`
(iii) `Ag_2SO_4 overset(Delta)-> 2Ag + SO_2 + O_2`
(iv) `Ag_2S_2O_3 + H_2O oversettext(Delta) -> Ag_2S + H_2SO_4`
`AgCl. AgBr. AgI` (but not `Ag_2S` ) are soluble in `Na_2S_2O_3` forming `[Ag(S_2O_3)_2]^(-3)` complexes
`AgBr : AgNO_3 overset(KBr) -> AgBr downarrow + KNO_3`
`text(Heating effect)` :
`2AgNO_3 overset(212^oC)-> 2AgNO_2 + O_2`
`2AgNO_2 overset(500^oC) -> 2Ag + 2NO + O_2`
`text(Other reactions)` : See fig.2.
`Ag_2O + H_2O_2 -> 2Ag + H_2O + O_2`
`K_2S_2O_8 + 2AgNO_3 + 2H_2O -> 2AgO + 2KHSO_4 + 2HNO_3`
`AgO` supposed to be paramagnetic due to `d^9` configuration. But actually it is diamagnetic and exists as `Ag^I[Ag^(III)O_2 ]`
`text(Reaction involved in developer)` :
`K_2Fe^(II)(C_2O_4)_2 + AgBr -> KFe^(III) (C_2O_4)_2 + Ag downarrow + KBr`