Potassium Dichromate(`K_2Cr_2O_7`) :
`(a)` `text(Preparation)` :
(i) It is prepared from Chromite ore `(FeCr_2O_4)`.
(ii) The preparation of `K_2Cr_2O_7` from chromite ore involve the following steps:
`(A)` `text(Conversion of Chromite ore to Sodium Chromate)` :
(i) The Chromite ore is fused with Sodium hydroxide or Sodium Carbonate in the presence of air.
`4FeCr_2O_4 + 16 NaOH + 7O_2 -> 8Na_2CrO_4 + 2Fe_2O_3 + 8 H_2O`
`4 FeCr_2O_4 + 8 Na_2CO_3 + 7O_2 -> 8 Na_2CrO_4 + 2Fe_2O_3 + 8 CO_2`
Sod. Chromite is extracted with water & ferric oxide is left behind.
`(B)` `text(Conversion of Sodium chromate to Sodium dichromate)` :
(i) The Sod. chromite is acidified with dilute `H_2SO_4` giving its dichromate.
`2 Na_2CrO_4 + H_2SO_4 -> Na_2Cr_2O_7 + Na_2SO_4 + H_2O`
(ii) On concentration, the less soluble sulphate crystallises and is filtered out. The resulting solution contains Sod. dichromate.
`(C)` `text(Conversion of Sod. dichromate to Pot. dichromate)` :
(i) Hot concentrated solution of `Na_2Cr_2O_7` with `KCl` in equimolar proportion
`Na_2Cr_2O_7 + 2 KCl -> K_2Cr_2O_7 + 2 NaCl`
`(b)` `text(Physical Properties)` :
It is orange- red crystalline compound having melting point `670` `K`.
`(c)` `text(Chemical Properties of)` `K_2Cr_2O_7` :
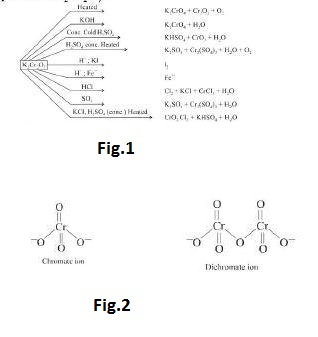
(i) See fig.1
(ii) `text(Chromyl Chloride Test)` :
(A) This is the test of Chloride
`K_2Cr_2O_7 + 2H_2SO_4 -> 2 KHSO_4 + 2CrO_3 + H_2O`
`[NaCl + H_2SO_4 -> NaHSO_4 + HCl] xx 4`
`[CrO_3 + 2HCl -> CrO_2Cl_2 + H_2 O] xx 2`
`=> (text(Total Reaction))` `K_2Cr_2O_7 + 6H_2SO_4 + 4NaCl -> 2 KHSO_4 + 4 NaHSO_4 + 2CrO_2Cl_2 + 3H_2O`
(B) When Chromyl Chloride vapours are passed through `NaOH` solution, yellow coloured solution is obtained.
`4 NaOH +CrO_2 Cl_2 -> Na_2 Cr O_4 + 2NaCl +H_2O`
(iii) `text(Action with HCl)` :
`K_2Cr_2O_7 + 14 HCl -> 2 KCl + 2CrCl_3 + 7H_2O + undersettext(Chlorine)(3Cl_2) uparrow`
`(iv)` `text(Oxidising character)` :
(A) The dichromates act. as powerful oxidizing agent in acidic medium.
`K_2Cr_2O _7+ 4 H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4 H_2O + undersettext(Nascent oxygen) (3 [O])`
(B) In term of electronic concept, the `Cr_2O_7^(2-)` ion take up electron in Acidic medium and hence acts as an oxidizing agent.
`underset(+6)[Cr_2O_7^(2-)] + 14 H^(+) + 6 e^(-) -> undersettext(+3)[2Cr^(3+)] + 7H_2O`
`text(Note)` : Both `Na_2Cr_2O_7` & `K_2Cr_2O_7` are oxidizing agents but `K_2Cr_2O_7` is preferred since it is not hygroscopic and can be used as primary standard.
(C) Some oxidizing reactions of `K_2Cr_2O_7` are:
It liberate `I_2` from `Kl`
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 ( SO_4)_3 + 4 H_2O + 3O`
`[2 KI + H_2SO_4+ O -> K_2SO_4 + H_2O + I_2 ] xx 3`
`K_2 Cr_2O_7+ 6 KI + 7H_2SO_4 -> 4K_2SO_4 + Cr_2(SO_4)_3 + 7 H_2O + 3I_2` (Iodine)
`text(Total Reaction)` `Cr_2O_7^(2-) + 14 H^+ + 6I^(-) + 6e^(-) -> 2 Cr^(3+) + 7H_2O + 3 I_2 + 6e^-`
or `Cr_2O_7^(2-) + 14H^+ + 6I^(-) -> 2Cr^(3+) + 3I_2 + 7H_2O`
It oxidises ferrous salts to ferric salts.
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4H_2O + 3(O)`
`2 [FeSO_4 + H_2SO_4 + O -> Fe_2(SO_4)_3 + H_2O] xx 3`
`text(Total Reaction)` `K_2Cr_2O_7 + 7H_2SO_4 + 6FeSO_4 -> 3Fe_2(SO_4)_3 + K_2SO_4 + Cr_2(SO_4)_3 + 7H_2O`
or `6Fe^(2+) + Cr_2O_7^(2-) + 14 H^+ -> 6Fe^(3+) + 2Cr^(3+) + 7H_2O`
It oxidises hydrogen sulphide to sulphur
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2(SO_4)_3 + 4H_2O + 3 [O]`
`[H_2S + O -> H_2 O + S] xx 3`
`text(Total Reaction)` `=>K_2Cr_2O_7 + 4H_2SO_4 + H_2S -> K_2SO_4 + Cr_2(SO_4)_3, + 3S + 7 H_2O`
or `H_2S + Cr_2O_7^(-2) + 8H^+ -> 2Cr^(3+) + 3S + 7H_2O`
It oxidises sulphites to sulphates
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4H_2O + 3(O)`
`[Na_2SO_3 + O -> Na_2SO_4] xx 3`
`text(Total Reaction)` `=> 3 Na_2SO_3 +K_2Cr_2O_7 + 4 H_2SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 + 3Na_2 SO_4 + 4H_2O`
or `3SO_3^(2-) + Cr_2O_7^(2-) + 8 H^+ -> 3 SO_4^(2-) + 2 Cr^(3+) + 4H_2O`
`SO_2` is oxidised to `H_2SO_4`
`K_2Cr_2O_7 + 4 H_2SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 + [O]`
`[SO_2 +(O) + H_2O -> H_2SO_4] xx 3`
`text(Total Reaction) ` `=> K_2Cr_2O_7 + H_2SO_4 + 3SO_2 -> Cr_2(SO_4)_3 H_2O`
or `Cr_2O_7^(2-) + 3 SO_2 + 2H^(+) -> 2 Cr ^(3+) + 3 (SO_4)^(2-) + H_2 O`
`text(NOTE)` :
`K_2Cr_2O_7 + H_2SO_4 + 3SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 +H_2O`
`undersettext(Chrome Alum)(K_2SO_4Cr_2(SO_4)_3 . 24 H_2O)`
Similarly, it oxidises, chlorides to chlorine, nitrites to nitrates, arsenites to arsenates thiosulphate to sulphate and sulphur `(S_2O_3^(2-) + O -> SO_4^(2-)+ S)` , `HBr` to `Br_2`, `Hl` to `I_2` .
It oxidises ethyl alcohol to acetaldehyde and acetic acid
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2(SO_4)_3 + 4H_2O + 3 [O]`
`undersettext(Ethyl Alcohol)(CH_3CH_2OH) + [O] -> CH_3CHO + H_2O`
`undersettext(Acetaldehyde)(CH_3CHO) + O -> CH_3COOH`
`text(Structure of chromate and dichromate ions)` : See fig.2.
`text(Uses)` : Potassium dichromate is used :
(i) As a volumetric reagent in laboratory for the estimation of ferrous ions, iodide ions etc.
(ii) For the preparation of chrome yellow `(PbCrO_4)`, Chrome red `(PbCrO_4.PbO)` , Zinc yellow `(ZnCrO_4)`, Gugrets green `(Cr_2O_3. 2H_2O)`, chromic acid `(CrO_3^-` orange), `K_3 [CrO_8]` (Red brown).
(iii) In organic chemistry as oxidising agents.
(iv) In photography for hardening gelatine films.
(i) It is prepared from Chromite ore `(FeCr_2O_4)`.
(ii) The preparation of `K_2Cr_2O_7` from chromite ore involve the following steps:
`(A)` `text(Conversion of Chromite ore to Sodium Chromate)` :
(i) The Chromite ore is fused with Sodium hydroxide or Sodium Carbonate in the presence of air.
`4FeCr_2O_4 + 16 NaOH + 7O_2 -> 8Na_2CrO_4 + 2Fe_2O_3 + 8 H_2O`
`4 FeCr_2O_4 + 8 Na_2CO_3 + 7O_2 -> 8 Na_2CrO_4 + 2Fe_2O_3 + 8 CO_2`
Sod. Chromite is extracted with water & ferric oxide is left behind.
`(B)` `text(Conversion of Sodium chromate to Sodium dichromate)` :
(i) The Sod. chromite is acidified with dilute `H_2SO_4` giving its dichromate.
`2 Na_2CrO_4 + H_2SO_4 -> Na_2Cr_2O_7 + Na_2SO_4 + H_2O`
(ii) On concentration, the less soluble sulphate crystallises and is filtered out. The resulting solution contains Sod. dichromate.
`(C)` `text(Conversion of Sod. dichromate to Pot. dichromate)` :
(i) Hot concentrated solution of `Na_2Cr_2O_7` with `KCl` in equimolar proportion
`Na_2Cr_2O_7 + 2 KCl -> K_2Cr_2O_7 + 2 NaCl`
`(b)` `text(Physical Properties)` :
It is orange- red crystalline compound having melting point `670` `K`.
`(c)` `text(Chemical Properties of)` `K_2Cr_2O_7` :
(i) See fig.1
(ii) `text(Chromyl Chloride Test)` :
(A) This is the test of Chloride
`K_2Cr_2O_7 + 2H_2SO_4 -> 2 KHSO_4 + 2CrO_3 + H_2O`
`[NaCl + H_2SO_4 -> NaHSO_4 + HCl] xx 4`
`[CrO_3 + 2HCl -> CrO_2Cl_2 + H_2 O] xx 2`
`=> (text(Total Reaction))` `K_2Cr_2O_7 + 6H_2SO_4 + 4NaCl -> 2 KHSO_4 + 4 NaHSO_4 + 2CrO_2Cl_2 + 3H_2O`
(B) When Chromyl Chloride vapours are passed through `NaOH` solution, yellow coloured solution is obtained.
`4 NaOH +CrO_2 Cl_2 -> Na_2 Cr O_4 + 2NaCl +H_2O`
(iii) `text(Action with HCl)` :
`K_2Cr_2O_7 + 14 HCl -> 2 KCl + 2CrCl_3 + 7H_2O + undersettext(Chlorine)(3Cl_2) uparrow`
`(iv)` `text(Oxidising character)` :
(A) The dichromates act. as powerful oxidizing agent in acidic medium.
`K_2Cr_2O _7+ 4 H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4 H_2O + undersettext(Nascent oxygen) (3 [O])`
(B) In term of electronic concept, the `Cr_2O_7^(2-)` ion take up electron in Acidic medium and hence acts as an oxidizing agent.
`underset(+6)[Cr_2O_7^(2-)] + 14 H^(+) + 6 e^(-) -> undersettext(+3)[2Cr^(3+)] + 7H_2O`
`text(Note)` : Both `Na_2Cr_2O_7` & `K_2Cr_2O_7` are oxidizing agents but `K_2Cr_2O_7` is preferred since it is not hygroscopic and can be used as primary standard.
(C) Some oxidizing reactions of `K_2Cr_2O_7` are:
It liberate `I_2` from `Kl`
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 ( SO_4)_3 + 4 H_2O + 3O`
`[2 KI + H_2SO_4+ O -> K_2SO_4 + H_2O + I_2 ] xx 3`
`K_2 Cr_2O_7+ 6 KI + 7H_2SO_4 -> 4K_2SO_4 + Cr_2(SO_4)_3 + 7 H_2O + 3I_2` (Iodine)
`text(Total Reaction)` `Cr_2O_7^(2-) + 14 H^+ + 6I^(-) + 6e^(-) -> 2 Cr^(3+) + 7H_2O + 3 I_2 + 6e^-`
or `Cr_2O_7^(2-) + 14H^+ + 6I^(-) -> 2Cr^(3+) + 3I_2 + 7H_2O`
It oxidises ferrous salts to ferric salts.
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4H_2O + 3(O)`
`2 [FeSO_4 + H_2SO_4 + O -> Fe_2(SO_4)_3 + H_2O] xx 3`
`text(Total Reaction)` `K_2Cr_2O_7 + 7H_2SO_4 + 6FeSO_4 -> 3Fe_2(SO_4)_3 + K_2SO_4 + Cr_2(SO_4)_3 + 7H_2O`
or `6Fe^(2+) + Cr_2O_7^(2-) + 14 H^+ -> 6Fe^(3+) + 2Cr^(3+) + 7H_2O`
It oxidises hydrogen sulphide to sulphur
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2(SO_4)_3 + 4H_2O + 3 [O]`
`[H_2S + O -> H_2 O + S] xx 3`
`text(Total Reaction)` `=>K_2Cr_2O_7 + 4H_2SO_4 + H_2S -> K_2SO_4 + Cr_2(SO_4)_3, + 3S + 7 H_2O`
or `H_2S + Cr_2O_7^(-2) + 8H^+ -> 2Cr^(3+) + 3S + 7H_2O`
It oxidises sulphites to sulphates
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2 (SO_4)_3 + 4H_2O + 3(O)`
`[Na_2SO_3 + O -> Na_2SO_4] xx 3`
`text(Total Reaction)` `=> 3 Na_2SO_3 +K_2Cr_2O_7 + 4 H_2SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 + 3Na_2 SO_4 + 4H_2O`
or `3SO_3^(2-) + Cr_2O_7^(2-) + 8 H^+ -> 3 SO_4^(2-) + 2 Cr^(3+) + 4H_2O`
`SO_2` is oxidised to `H_2SO_4`
`K_2Cr_2O_7 + 4 H_2SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 + [O]`
`[SO_2 +(O) + H_2O -> H_2SO_4] xx 3`
`text(Total Reaction) ` `=> K_2Cr_2O_7 + H_2SO_4 + 3SO_2 -> Cr_2(SO_4)_3 H_2O`
or `Cr_2O_7^(2-) + 3 SO_2 + 2H^(+) -> 2 Cr ^(3+) + 3 (SO_4)^(2-) + H_2 O`
`text(NOTE)` :
`K_2Cr_2O_7 + H_2SO_4 + 3SO_4 -> K_2SO_4 +Cr_2(SO_4)_3 +H_2O`
`undersettext(Chrome Alum)(K_2SO_4Cr_2(SO_4)_3 . 24 H_2O)`
Similarly, it oxidises, chlorides to chlorine, nitrites to nitrates, arsenites to arsenates thiosulphate to sulphate and sulphur `(S_2O_3^(2-) + O -> SO_4^(2-)+ S)` , `HBr` to `Br_2`, `Hl` to `I_2` .
It oxidises ethyl alcohol to acetaldehyde and acetic acid
`K_2Cr_2O_7 + 4H_2SO_4 -> K_2SO_4 + Cr_2(SO_4)_3 + 4H_2O + 3 [O]`
`undersettext(Ethyl Alcohol)(CH_3CH_2OH) + [O] -> CH_3CHO + H_2O`
`undersettext(Acetaldehyde)(CH_3CHO) + O -> CH_3COOH`
`text(Structure of chromate and dichromate ions)` : See fig.2.
`text(Uses)` : Potassium dichromate is used :
(i) As a volumetric reagent in laboratory for the estimation of ferrous ions, iodide ions etc.
(ii) For the preparation of chrome yellow `(PbCrO_4)`, Chrome red `(PbCrO_4.PbO)` , Zinc yellow `(ZnCrO_4)`, Gugrets green `(Cr_2O_3. 2H_2O)`, chromic acid `(CrO_3^-` orange), `K_3 [CrO_8]` (Red brown).
(iii) In organic chemistry as oxidising agents.
(iv) In photography for hardening gelatine films.